Application of dihydromyricetin in preparing drugs for treating iron overload diseases
A technology for dihydromyricetin and disease treatment, applied in metabolic diseases, drug combinations, pharmaceutical formulations, etc., can solve the problem that polyphenols do not have the expected effect and other problems, and achieve the effect of protecting cells from damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
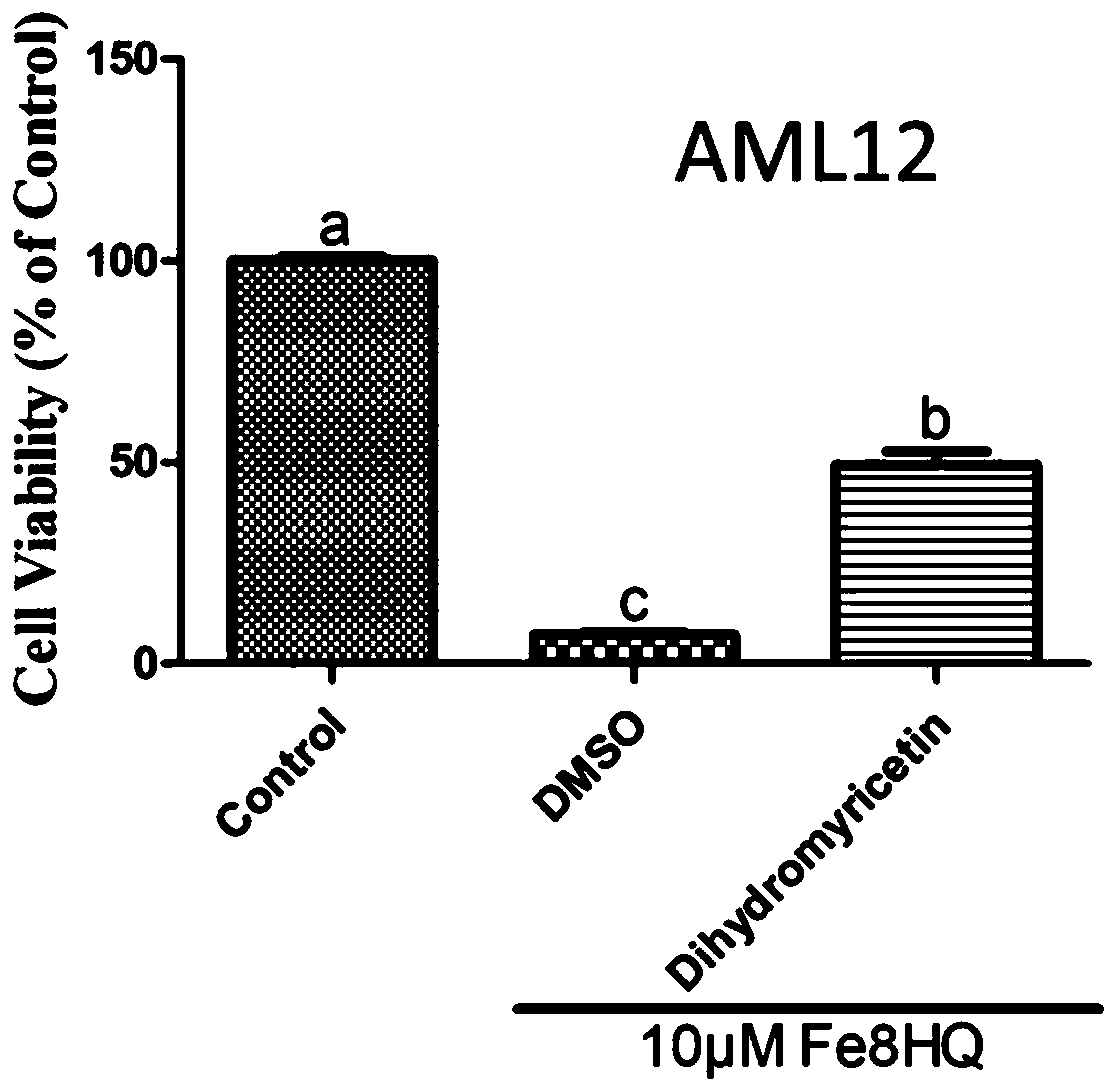
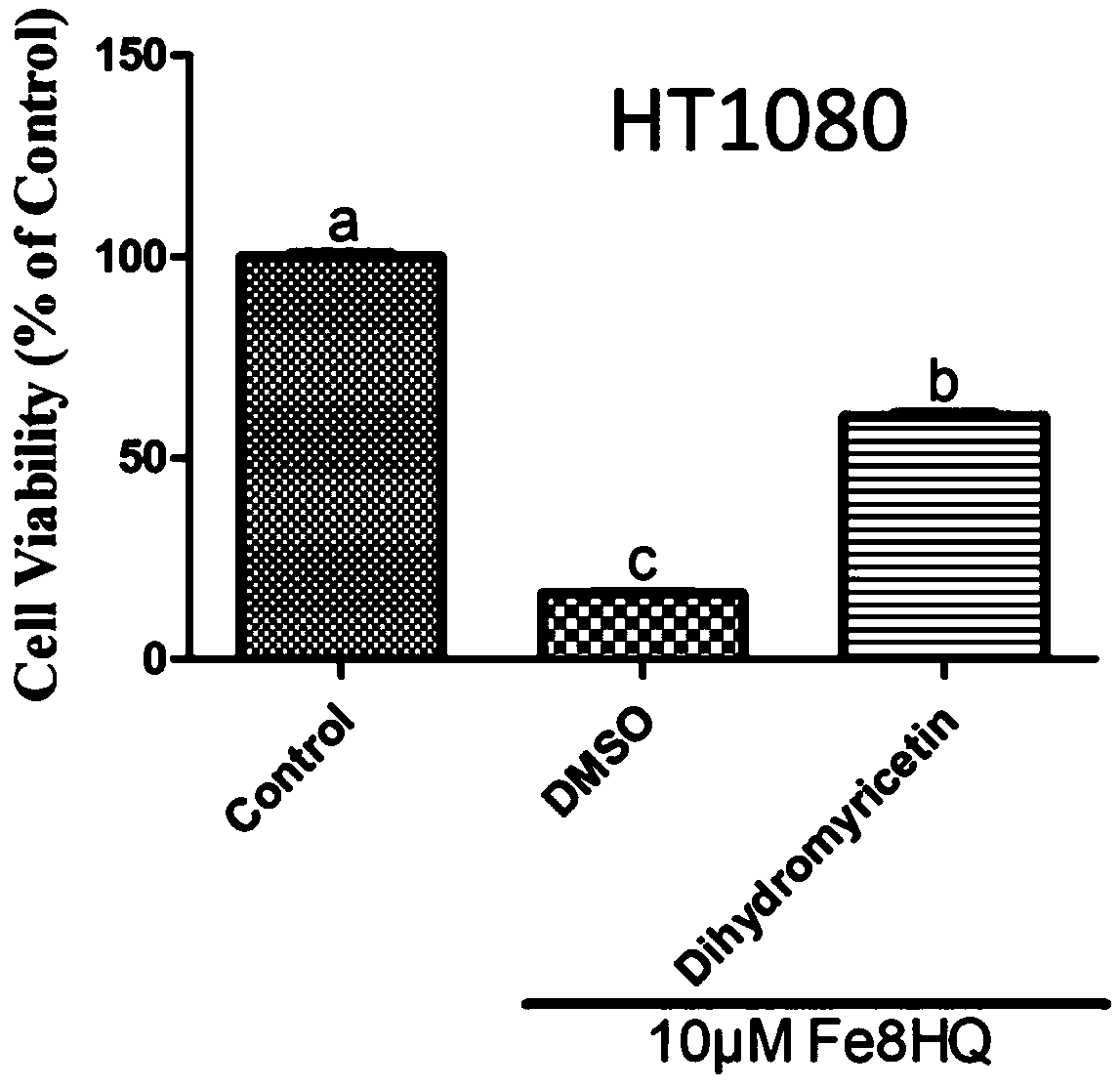
[0037] Example 1: Cell Viability Detection
[0038] 1) Digest and centrifuge the HT1080 cells and AML12 cells grown to 80%-90% confluence, and dilute them at 1×10 per well 4 The number of cells was inoculated into a 96-well cell culture plate and cultivated for 24 hours;
[0039]2) Add control and 10 μM dihydromyricetin to the 96-well plate containing the cells; then add Fe-8HQ 10 μM to each well plate for 4 hours, and set 6 replicates in each group;
[0040] 3) At 37°C, 5% CO 2 Conditioned cell culture incubator;
[0041] 4) Carefully pipette the treatment solution in the 96-well plate, and add the cell culture medium containing 10% CCK-8 cell viability detection reagent prepared in advance, and continue to store at 37°C, 5% CO 2 Cultivate in a conditioned cell culture incubator for 1 to 4 hours;
[0042] 5) Cell activity was detected on the SpectraMax M5 microplate detection system, and the detection parameter of CCK-8 was 450nm.
[0043] 6) Calculate the cell viability...
Embodiment 2
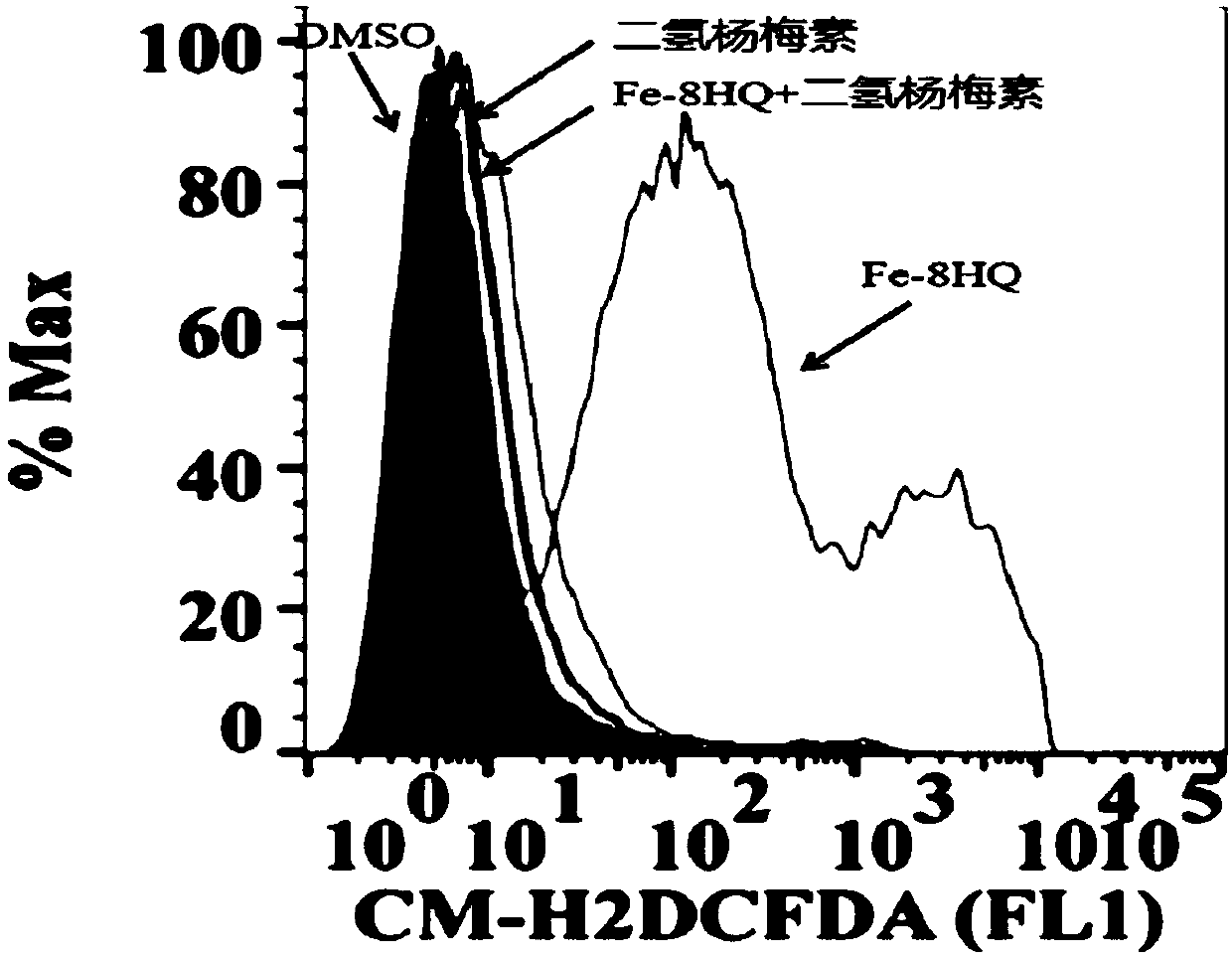
[0045] Example 2: Determination of cytoplasmic reactive oxygen species ROS.
[0046] There are various ROS in cells, which can be stained by specific reagents, among which cytoplasmic ROS can be stained with CM-H2DCFDA.
[0047] 1) The day before the test, human fibroblastosarcoma carcinoma HT1080 cells grown to 80%-90% confluence were digested and centrifuged, and 2×10 5 The number of cells, inoculated into 6-well cell culture plate, and the cells were grown for 24 hours;
[0048] 2) Treat cells with DMSO, 10 μM Fe-8HQ, 10 μM Fe-8HQ+10 μM dihydromyricetin, 10 μM dihydromyricetin for 4 hours;
[0049] 3) After the treatment time is up, discard the culture medium in the six-well plate, gently wash twice with PBS, add 600 μL of Accutase cell digestion solution to each well to digest the cells, then add the medium to stop the digestion, gently pipette and collect the cells, and rotate at 2500 rpm Centrifuge for 5 minutes;
[0050] 4) After the supernatant was discarded, 500 μL...
Embodiment 3
[0052] Example 3: Determination of cellular mitochondrial reactive oxygen species ROS.
[0053] There are various ROS in cells, which can be stained by specific reagents, among which mitochondrial ROS can be stained by MitoSOX Red.
[0054] 1) The day before the test, human fibroblastosarcoma carcinoma HT1080 cells grown to 80%-90% confluence were digested and centrifuged, and 2×10 5 The number of cells, inoculated into 6-well cell culture plate, and the cells were grown for 24 hours;
[0055] 2) Treat the cells with DMSO, 10 μM Fe-8HQ, 10 μM Fe-8HQ+10 μM dihydromyricetin, 10 μM dihydromyricetin for 4 hours;
[0056] 3) After the treatment time is up, discard the culture medium in the six-well plate, gently wash twice with PBS, add 600 μL of Accutase cell digestion solution to each well to digest the cells, then add the medium to stop the digestion, gently pipette and collect the cells, and rotate at 2500 rpm Centrifuge for 5 minutes;
[0057] 4) After discarding the supern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com