ATP (adenosine triphosphate) multisite binding fluorescence-enhanced type probe molecule and preparation method and application thereof
A technology of fluorescent molecular probes and derivatives, applied in the field of fluorescent probes, can solve the problems of high response concentration and poor mitochondrial targeting, and achieve the effects of simple synthesis method, easy large-scale synthesis, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for synthesizing an ATP fluorescent probe containing a secondary amine segment and a rhodamine derivative (Rh-NH-PBA) modified with phenylboronic acid, the specific preparation steps of which are:
[0042] (1) Add 1.0mmol Rhodamine 6G (0.48g) into a 100mL three-neck flask filled with 15mL of absolute ethanol and stir to dissolve completely, then add 1g of diethylenetriamine (10.0mmol) dropwise, and stir and reflux at 90°C for 32h , until the color of the solution becomes lighter, the reaction is terminated, and the solvent is removed by rotary evaporation after cooling to room temperature, then water and dichloromethane are added for extraction and washing 2 times (20mL water and 30mL dichloromethane are shared), and organic matter after 2 extractions and washings is collected. phase, and the solvent was removed by rotary evaporation, and the mixture of dichloromethane and ethanol with a volume ratio of 6:0.2 to 6:0.8 (gradient elution, adjust the volume ratio of ...
Embodiment 2
[0047] A method for synthesizing an ATP fluorescent probe containing a secondary amine segment and a rhodamine derivative (Rh-NH-PBA) modified with phenylboronic acid, the specific preparation steps of which are:
[0048] (1) Add 1.0mmol Rhodamine B (0.48g) into a 100mL three-necked flask filled with 15mL of absolute ethanol and stir to dissolve completely, then add 1g of diethylenetriamine (10.0mmol) dropwise, and stir and reflux at 90°C for 32h , until the color of the solution becomes lighter, the reaction is terminated, and the solvent is removed by rotary evaporation after cooling to room temperature, then water and dichloromethane are added for extraction and washing 3 times (20mL water and 30mL dichloromethane are shared), and the organic matter after 3 times of extraction and washing is collected. phase, and the solvent was removed by rotary evaporation, and the mixture of dichloromethane and ethanol with a volume ratio of 6:0.2 to 6:0.8 (gradient elution, adjust the vo...
Embodiment 3
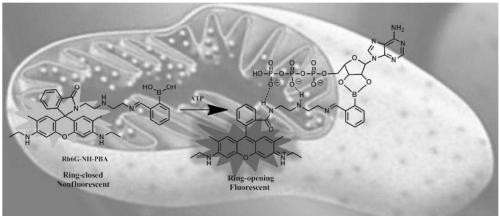
[0053] Rhodamine derivatives Rh6G-NH-PBA and RhB-NH-PBA containing secondary amine segments and phenylboronic acid modification prepared in Examples 1 and 2 were used as ATP fluorescent probes for research.
[0054] Rh6G-NH-PBA and RhB-NH-PBA were respectively dissolved in EtOH to prepare Rh6G-NH-PBA stock solution and RhB-NH-PBA stock solution with a concentration of 1 mM. Dissolve adenosine triphosphate (ATP), guanosine monophosphate (GMP), sodium pyrophosphate (SDP), adenosine-5-phosphate (AMP), uridine 5'-triphosphate (UTP) in distilled water , adenosine (ADS), sodium dihydrogen phosphate (H 2 PO 4 - ), disodium hydrogen phosphate (HPO 4 2- ), sodium phosphate (PO 4 3- ), sodium chloride (Cl - ), sodium sulfate (SO 4 2- ), sodium nitrate (NO 3 - ), sodium carbonate (CO 3 2- ) to make a stock solution with a concentration of 10 mM. A certain volume (can be 0, that is, no addition) of stock solution of at least one analyte was added to 50 μL Rh6G-NH-PBA stock s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com