Evaluation method for acute toxicity of mesenchymal stem cell preparation and application
An evaluation method and stem cell technology, applied in the biological field, can solve problems such as lack of technical means for acute toxicity, and achieve the effect of reducing the risk of inaccurate results and avoiding inaccurate evaluation results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
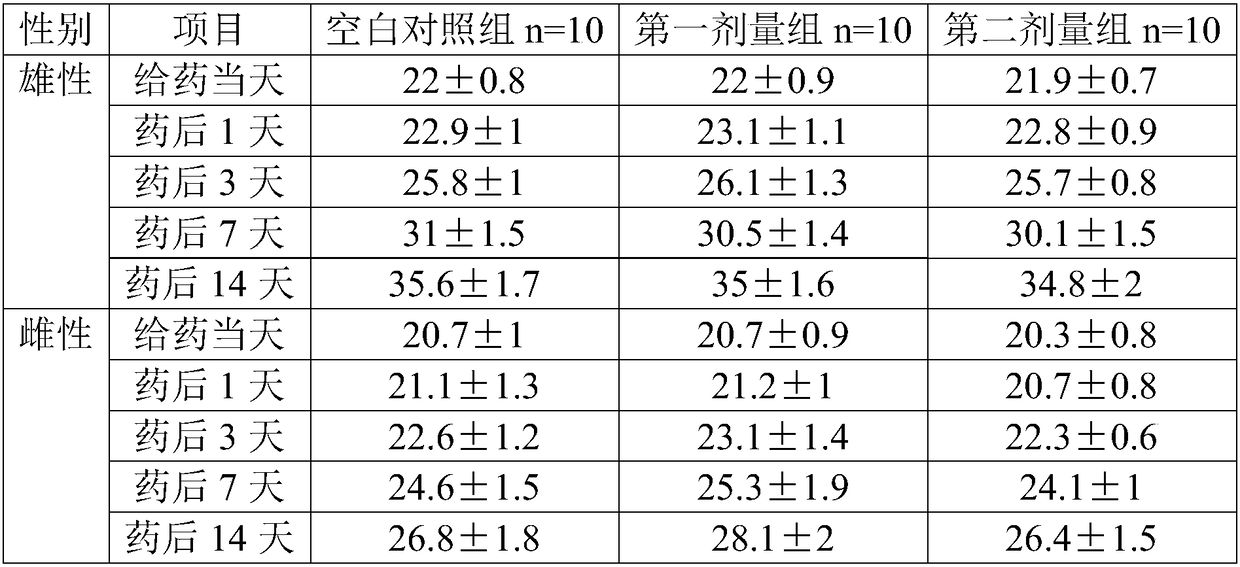
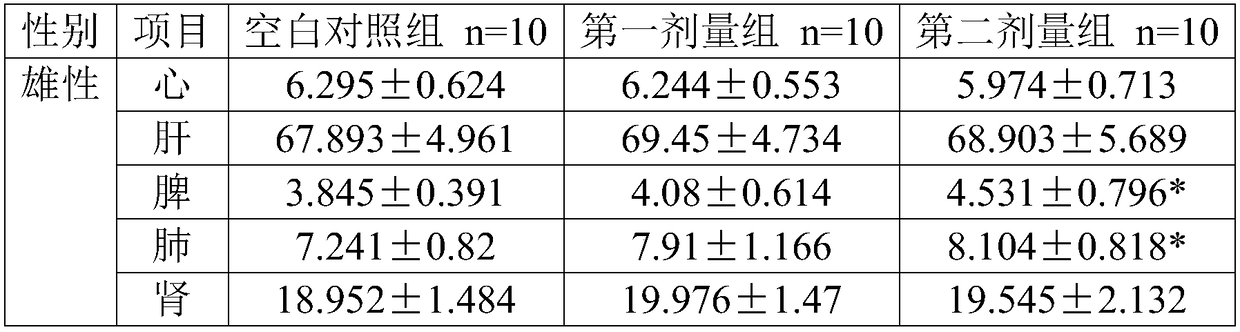
[0067] This example provides a method for evaluating the acute toxicity of human umbilical cord mesenchymal stem cells.
[0068] experimental design:
[0069] (1) Group setting: The experiment set up 3 groups: 1 blank control group and 2 dosing groups. The dosing groups include the first dose group and the second dose group, each with 20 mice, half male and half.
[0070] (2) Metering design and basis: In the proposed clinical plan, the human dose is intravenously 6.0×10 7 cells / 100ml / person, converted into a clinical preparation concentration of 6.0×10 5 cells / ml, the clinical dose is 1.0×10 6 cells / kg (60kg for humans), 20g for mice, the clinical equivalent dose is 1.3×10 7 cells / kg.
[0071] In this embodiment, there are two administration groups, the first dose group and the second dose group.
[0072] The dose of the first dose group is 1.51×10 7 cells / kg, the drug concentration is 6.05×10 5 cells / ml, the administration volume is 25ml / kg, the dose of the first dose group is slightl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com