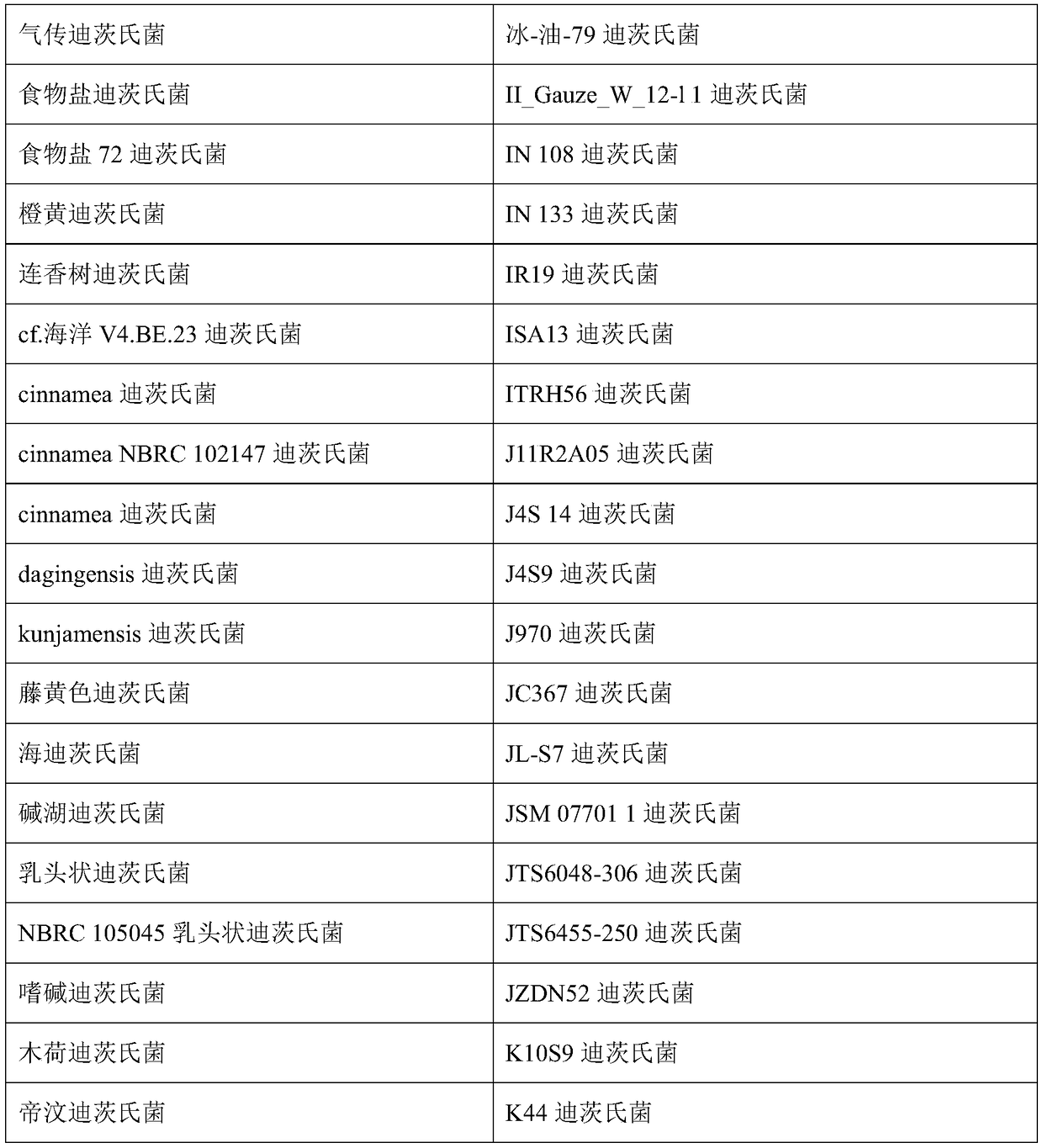
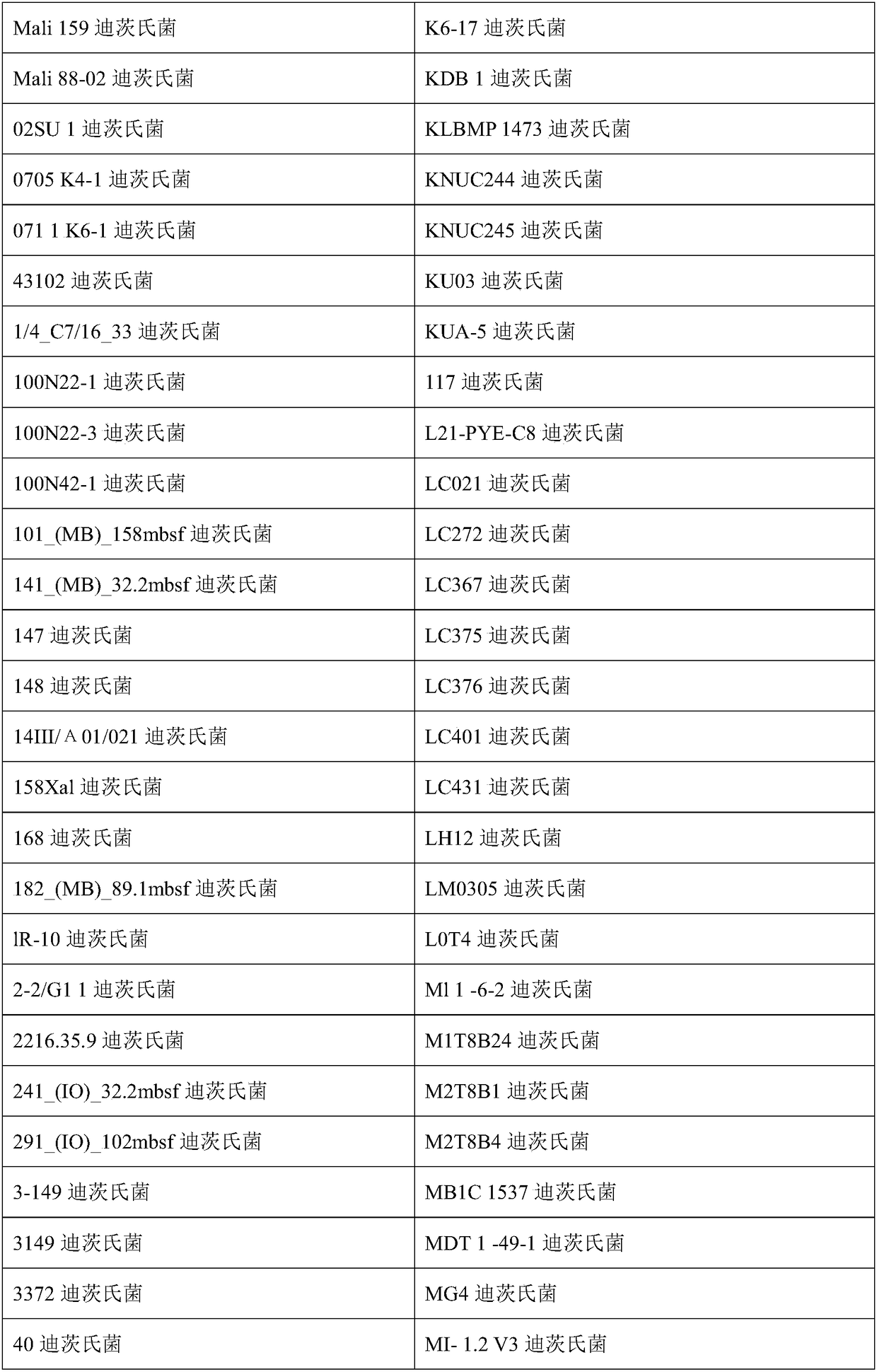
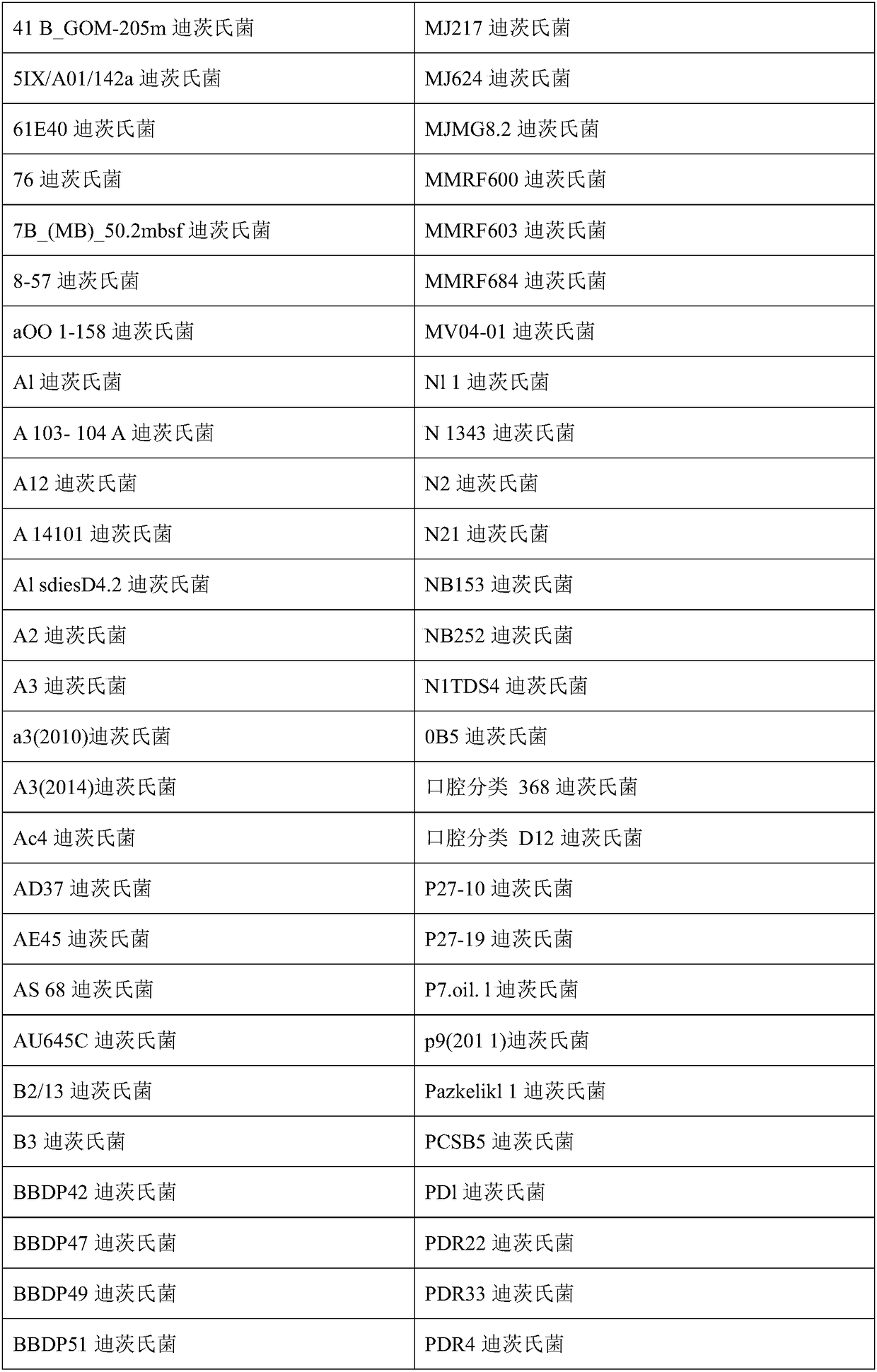
Compositions and methods of treatment of chronic infectious diseases
A kind of technology of composition and medicine, applied in the field of pharmaceutical composition of chronic disease, the field of pharmaceutical composition of infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Selection of MAP Intervention Bacteria from Cattle
[0050] The following examples describe exemplary methods for selecting therapeutic combinations of intervening bacteria to treat M. avium subsp. paratuberculosis (MAP) in cattle.
[0051] MAP can be grown from a variety of sources to cover a variety of cattle, preferably across many farms and many countries. Blood from these cows can be split into several different test tubes and various concentrations of (exemplary) Dietzella sp. from 10 2 to 10 15 Added to several tubes, but added saline vehicle only to control tubes. After 8 or 20 days of incubation, MAP proliferation within the stained macrophages was examined microscopically to see if a particular Dietzia species selected from a particular cow inhibited MAP. Microscopic screening assays for macrophage proliferation using MAPs save a considerable amount of time required for MAPs grown in culture.
[0052] The next stage is cultivation on slants sui...
Embodiment 2
[0054] Example 2: Selection of human anti-MAP intervention strains
[0055] The following examples describe exemplary methods for selecting therapeutic combinations of intervening bacteria to treat M. avium subsp. paratuberculosis (MAP) in humans.
[0056] Blood was collected from patients with Crohn's disease and cultured for 10-20 days with macrophage strains of MAP in the presence and absence of Dietzella or other candidate inhibitory strains, e.g., selected from Example 1 strains of the method described. Multiple Dietzia strains were tested for each patient, and then the most effective Dietzia inhibitors were combined in a panel of 6-10 Dietzia strains for use as oral treatments. The greater the number of human strains co-cultured with Dietzella, the greater the coverage of probiotic inhibition in Crohn's disease. Rising concentrations of Dietzella during blood incubation will also help determine the titer at which Dietzia will begin to inhibit infection with MAP. Her...
Embodiment 3
[0073] Example 3: Selection of MAP-intervention bacteria from sheep
[0074] The following examples describe exemplary methods for selecting therapeutic combinations of intervening bacteria to treat Mycobacterium avium subtype paratuberculosis (MAP) in sheep (Johne's disease of sheep).
[0075] The same exemplary procedure can be repeated in sheep in a similar manner as described for humans and cattle. However, in cattle and sheep, MAP can actually be grown in the lab, so an intervention to suppress bacteria can be tested on sheep and cattle MAP, and it can be more easily tested on human MAP, which would not be so easy Grow in laboratories using solid media or even liquid media.
[0076] In an alternative embodiment, methods for culturing ovine and bovine MAPs that are also suitable for the growth of M. tuberculosis, on HEYM slants and modified Middlebrook 7H10 agar medium can be used to produce MAP slants. Therefore, these media in screw cap tubes will contain M. paratube...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com