Crystal form of free base of imidazoisoindole derivatives and preparation method thereof
A crystal form and drug technology, which is applied in the field of crystal form of imidazoisoindole derivatives free base and its preparation, can solve the problems of poor product stability, difficult production scale-up, poor fluidity, etc., and achieve good crystal form stability , stable production process and high chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
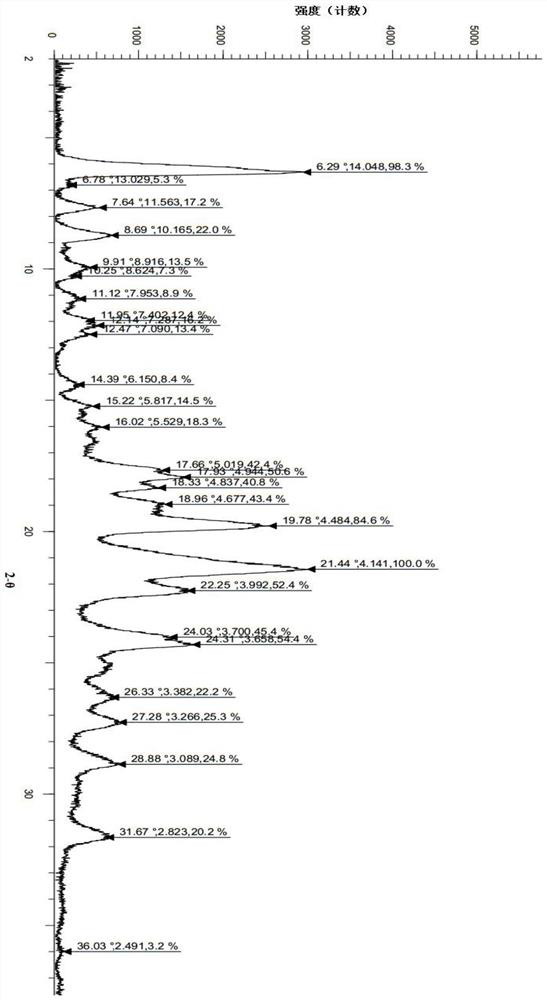
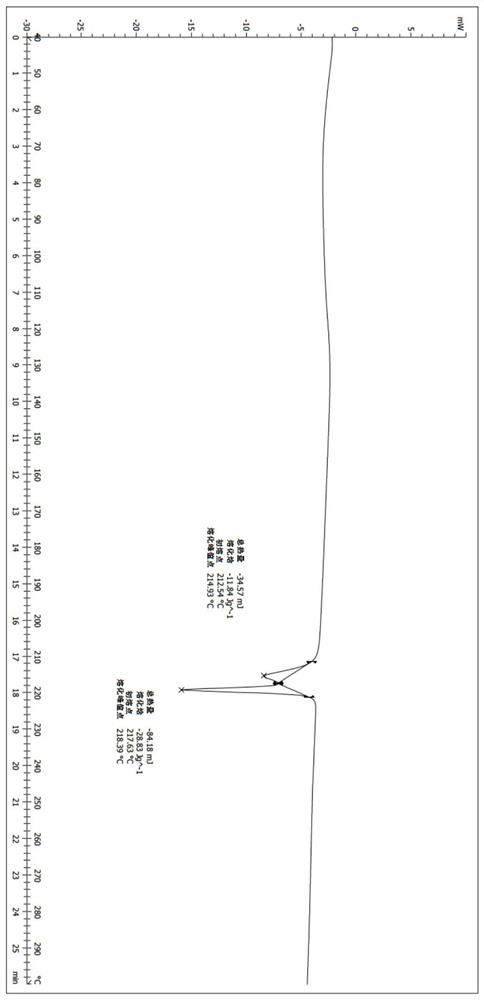
[0069] 300mg of (S)-2-(4-(4-(4-(6-fluoro-5H-imidazo[5,1-a]isoindol-5-yl)piperidin-1-yl)phenyl )-1H-pyrazol-1-yl)ethanol (prepared according to the method in Examples 40 and 41 in patent application WO2016169421) crude product was added to the reaction flask, and then 35 mL of dichloromethane was added, heated to reflux, and the solid was dissolved. Turn off the heat and stir for crystallization. The next day, it was filtered by suction and dried to obtain 238 mg of white solid with a yield of 79.3%. The X-ray diffraction pattern of this crystalline sample is shown in figure 1 , whose DSC spectrum is shown in figure 2 , define this crystal form as II crystal form, and its characteristic peak positions are shown in the table below:
[0070] Table 1. Characteristic peaks of crystal form II
[0071]
[0072]
Embodiment 2
[0074] 300mg of (S)-2-(4-(4-(4-(6-fluoro-5H-imidazo[5,1-a]isoindol-5-yl)piperidin-1-yl)phenyl )-1H-pyrazol-1-yl)ethanol crude product was added to the reaction flask, 15 mL of tetrahydrofuran was added, heated to reflux, the solid dissolved, turned off the heating, and stirred for crystallization. The next day, it was suction filtered and dried to obtain 264 mg of white solid with a yield of 88.0%. The crystalline sample was determined to be Form II by XRPD detection.
Embodiment 3
[0076] 300mg of (S)-2-(4-(4-(4-(6-fluoro-5H-imidazo[5,1-a]isoindol-5-yl)piperidin-1-yl)phenyl )-1H-pyrazol-1-yl)ethanol crude product was added to the reaction flask, ethylene glycol dimethyl ether 45mL was added, heated to reflux, the solid was dissolved, the heating was turned off, and the mixture was stirred for crystallization. The next day, it was filtered by suction and dried to obtain 185 mg of white solid with a yield of 61.7%. The crystalline sample was determined to be Form II by XRPD detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com