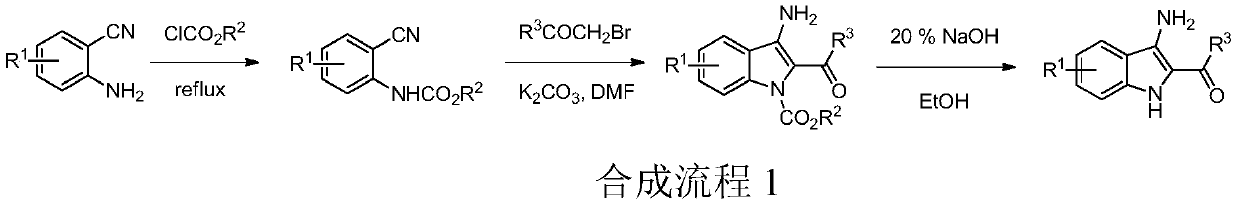
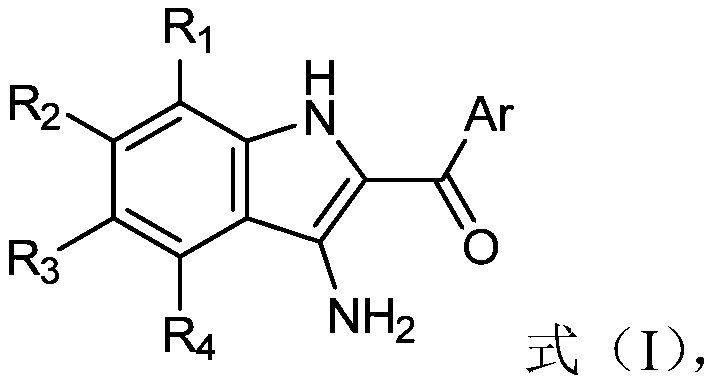
Preparation method and application of 2-acyl-3-aminoindole compounds
A compound and acyl technology, which is applied in the field of preparation of 2-acyl-3-aminoindole compounds, can solve the problems of low yield, cumbersome preparation process, and complicated operation, and achieve simple operation, mild reaction conditions, and increased application effect of possibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2-acyl-3-aminoindole:
[0048]
[0049] Add acetophenone (0.5mmol), iodine (0.8mmol) and 2mL of DMSO to a 38mL pressure tube, and after the first reaction at 100°C for 45min (the acetophenone completely disappeared), add 2 -Aminobenzonitrile (0.5mmol), NaHS·H 2 O (2.5mmol) was subjected to the second reaction at 100°C. After 4 hours, the reaction liquid was extracted with ethyl acetate, and the organic layer was washed with saturated sodium thiosulfate and saturated brine, dried over anhydrous sodium sulfate, and then decompressed. The solvent was evaporated to obtain a crude product, which was separated and purified by column chromatography with a mixture of petroleum ether and ethyl acetate to obtain a yellow oil with a total yield of 71%.
[0050] The obtained yellow oil is carried out to the proton nuclear magnetic spectrum test, and the obtained proton nuclear magnetic spectrogram result is:
[0051] ( 1 H-NMR (600MHz, CDCl 3 ,ppm):δ7.95(s,1H),7...
Embodiment 2
[0054] Synthesis of (3-amino-1hydro-indol-2-yl)(4-(methylsulfonyl)phenyl)methanone:
[0055]
[0056] The specific steps are: add 4-thiamphenicol acetophenone (0.5mmol), iodine (0.8mmol) and 2mL of DMSO to a 38mL pressure tube, and carry out the first reaction at 100°C for 60min (4-methylsulfone base acetophenone disappears completely), in the reaction liquid, add 2-aminobenzonitrile (0.5mmol), NaHS·H 2 O (2.5mmol) carried out the second reaction at 100°C. After 7 hours, the reaction solution was extracted with ethyl acetate, and the organic layer was washed with saturated sodium thiosulfate and saturated brine respectively, dried over anhydrous sodium sulfate, and reduced The crude product was obtained by pressure evaporation to remove the solvent, and the crude product was separated and purified by column chromatography with a mixture of petroleum ether and ethyl acetate to obtain a yellow solid with a total yield of 76%.
[0057] Gained yellow solid is carried out proto...
Embodiment 3
[0062] Synthesis of (3-amino-1hydro-indol-2-yl)(4-bromophenyl)methanone:
[0063]
[0064] The specific steps are: add 4-bromoacetophenone (0.5mmol), iodine (0.8mmol) and 2mL of DMSO to a 38mL pressure tube, and carry out the first reaction at 100°C for 60min (4-bromoacetophenone disappear completely), add 2-aminobenzonitrile (0.5mmol) to the reaction solution, NaHS·H 2 O (2.5mmol) was subjected to the second reaction at 100°C. After 4 hours, the reaction liquid was extracted with ethyl acetate, and the organic layer was washed with saturated sodium thiosulfate and saturated brine, dried over anhydrous sodium sulfate, and then decompressed. The solvent was evaporated to obtain a crude product, which was separated and purified by column chromatography with a mixture of petroleum ether and ethyl acetate to obtain a yellow solid with a total yield of 72%.
[0065] Gained yellow solid is carried out proton nuclear magnetic spectrum test and carbon nuclear magnetic spectrum tes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com