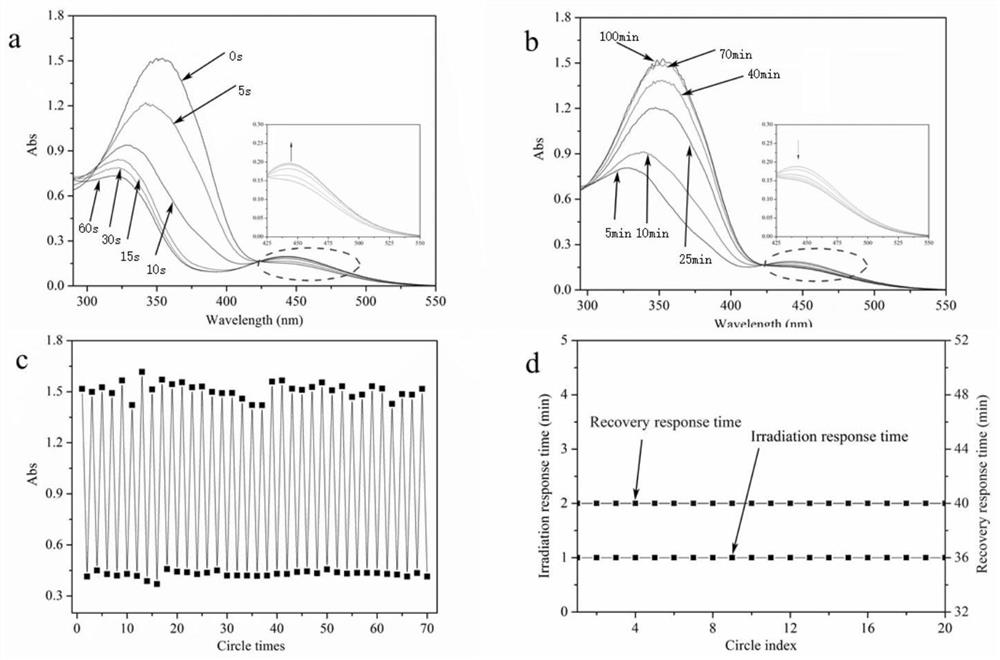
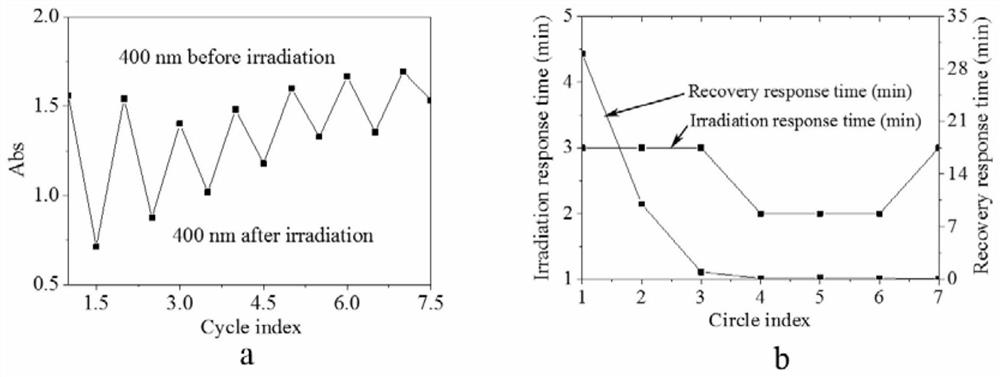
A functional polymer optical switch and its synthesis method
A synthetic method and polymer technology, applied in the field of new materials, can solve problems such as impact and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A method for synthesizing a functionalized polymer optical switch, comprising the following steps:
[0059] Step (1): Dissolve a certain amount of p-aminoazobenzene in dichloromethane in a dry environment with a concentration of 1.3M, and add acryloyl chloride dropwise to the system under ice bath conditions, acryloyl chloride: azobenzene The molar ratio of the solution was 1.1:1, and the dropping rate was 5mL / h. After reacting for 6 hours, an excess of ethylenediamine was added, washed several times with saturated brine to remove unreacted acid chloride and ethylenediamine, and the product was extracted with ethyl acetate. After pressure distillation and vacuum drying, pure double bond modified azobenzene derivatives were obtained.
[0060] Step (2): Dissolve a certain amount of N‐hydroxysuccinimide in dichloromethane in a dry environment with a concentration of 1.3M, and add acryloyl chloride dropwise to the system under ice bath conditions, acryloyl chloride: N ‐The...
Embodiment 2
[0063] A method for synthesizing a functionalized polymer optical switch, comprising the following steps:
[0064] Step (1): Dissolve a certain amount of p-hydroxyazobenzene in chloroform in a dry environment with a concentration of 0.1M, and add acryloyl chloride dropwise to the system under ice bath conditions, acryloyl chloride: azobenzene The molar ratio of the solution is 1.5:1, and the addition rate is 15mL / h. Add excess ethylenediamine after 12 hours of reaction, wash with saturated brine several times to remove unreacted acid chloride and ethylenediamine, and extract the product with ethyl acetate. After pressure distillation and vacuum drying, pure double bond modified azobenzene derivatives were obtained.
[0065] Step (2): Dissolve a certain amount of N‐hydroxysuccinimide in chloroform in a dry environment with a concentration of 0.1M, and add acryloyl chloride dropwise to the system under ice bath conditions, acryloyl chloride: N ‐The molar ratio of hydroxysuccini...
Embodiment 3
[0068] A method for synthesizing a functionalized polymer optical switch, comprising the following steps:
[0069] Step (1): Dissolve a certain amount of p-carboxyazobenzene in tetrahydrofuran in a dry environment with a concentration of 10M, add acryloyl chloride dropwise to the system under ice bath conditions, the molar ratio of acryloyl chloride: azobenzene The ratio is 1.2:1, and the dropping rate is 10mL / h. After 2 hours of reaction, excess ethylenediamine is added, washed several times with saturated brine to remove unreacted acid chloride and ethylenediamine, and the product is extracted with ethyl acetate, and after vacuum distillation Vacuum drying to obtain pure double bond modified azobenzene derivatives.
[0070] Step (2): Dissolve a certain amount of N-hydroxysuccinimide in tetrahydrofuran in a dry environment with a concentration of 10M, and add acryloyl chloride dropwise to the system under ice-bath conditions. Acryloyl chloride: N-hydroxysuccinate The molar r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com