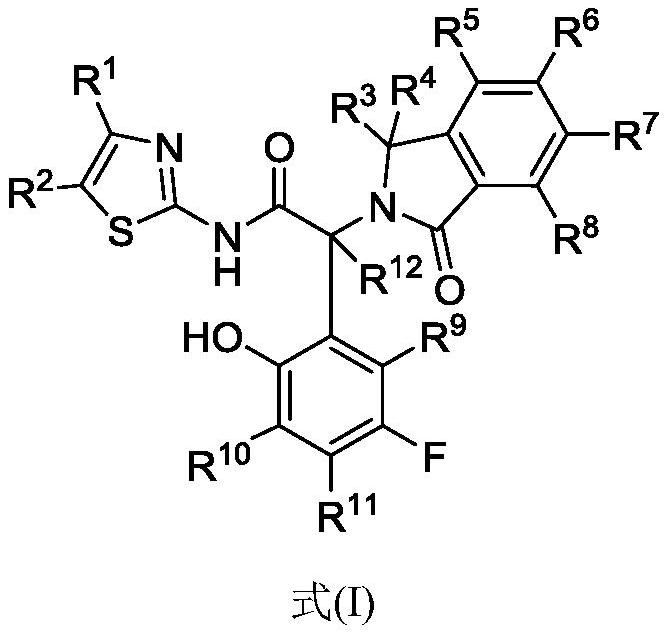
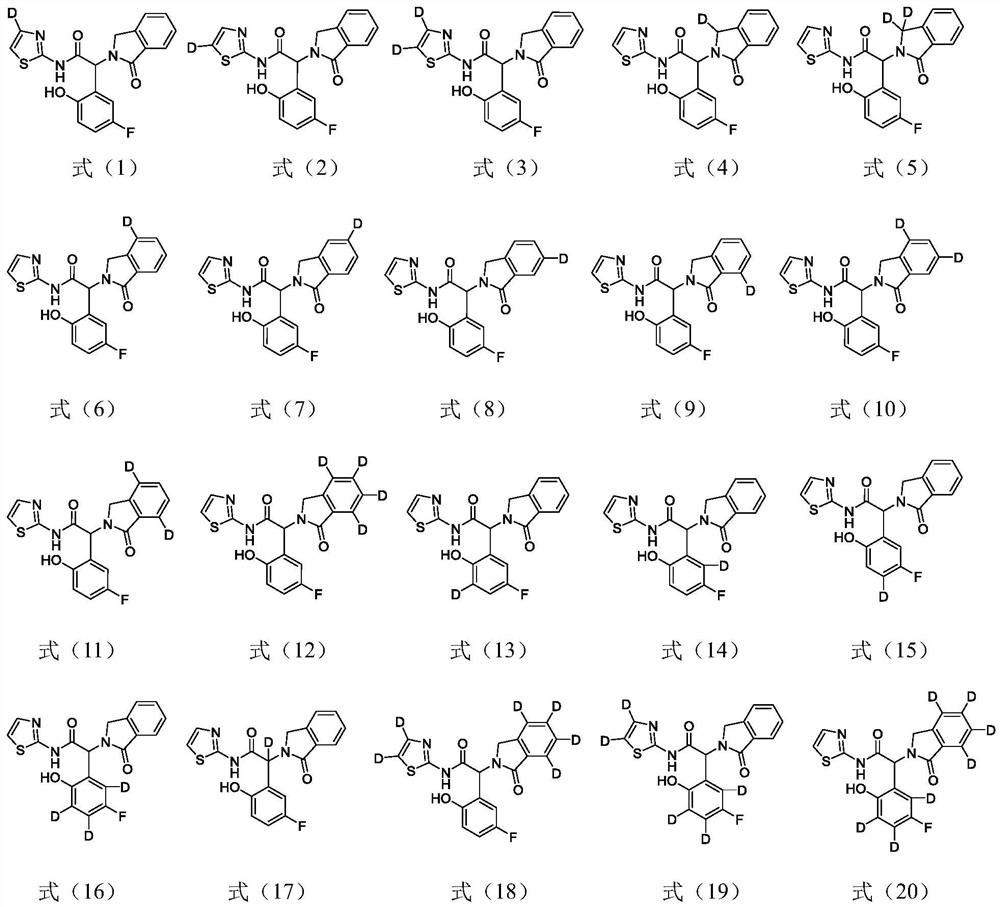
A kind of amide compound and composition comprising the same and use thereof
A technology of compounds and compositions, applied in the field of medicine, can solve problems such as acquired drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] The following synthetic route was used to prepare 2-(5-fluoro-2-hydroxyphenyl)-2-(1-oxoisoindoline-2-yl)-N-(thiazol-2-yl-5-d)ethyl Amide (compound 11), comprising the following steps:
[0116]
[0117] Step 1: Synthesis of compound 3.
[0118] Add 4mL of methanesulfonic acid to the reaction flask, cool to 0°C, add α-hydroxyhippuric acid (compound 1, 1g, 5.12mmol), add 4-fluoroanisole (646mg, 5.12mmol) at 0°C, and Under stirring for 1 hour, the reactant was slowly added dropwise to ice water, a white solid was washed out, filtered, the filter cake was washed with water, and vacuum-dried to obtain compound 3 (1.3 mg, yield 86.6%), LC-MS (APCI ):m / z=302(M-1) - .
[0119] Step 2: Synthesis of compound 4.
[0120]Add compound 3 (1g, 3.3mmol) to the reaction flask, add 60mL of 6N hydrochloric acid, heat up to 100°C, react for 36 hours, TLC (thin layer chromatography) detects that the raw materials have reacted completely, cool to room temperature, filter, and the filtr...
Embodiment 2
[0134] The following synthetic route was used to prepare 2-(5-fluoro-2-hydroxyphenyl)-2-(1-oxoisoindoline-2-yl-3-d)-N-(thiazol-2-yl)ethyl Amide (compound 21), comprising the following steps:
[0135]
[0136] Step 1: Synthesis of Compound 12.
[0137] Add compound 4 (2.08g, 1045mmol) to the reaction flask, add methanol 30mL, cool to 0°C, dropwise add thionyl chloride (1.5mL, 20.9mmol) and raise to 75°C after the dropwise addition, react for 3 hours, the raw material After the reaction was complete, concentrated to remove methanol and excess thionyl chloride, added 20 mL of methyl tert-butyl ether to make a slurry to obtain compound 12 (2.15 g, yield 98.4%), LC-MS (APCI): m / z=214 (M +1) + .
[0138] Step 2: Synthesis of Compound 14.
[0139] Add compound 13 (2g, 12mmol) to the reaction flask, add thionyl chloride 10mL at 0°C, reflux for 3 hours, concentrate to remove excess thionyl chloride to obtain compound 14 (2.74g, yield 100%).
[0140] Step 3: Synthesis of Compoun...
Embodiment 3
[0155] The following synthetic route was used to prepare 2-(5-fluoro-2-hydroxyphenyl)-2-(1-oxoisoindoline-2-y-yl-3,3-d 2 )-N-(thiazol-2-yl)acetamide (compound 25), comprising the following steps:
[0156]
[0157] Step 1: Synthesis of compound 22.
[0158] Add compound 16 (1g, 2.91mmol) to the reaction flask, add zinc powder (10g, 152mmol) in 5 times, add deuterated acetic acid 10mL, heat up to 110°C and react for 7 hours, TLC detects that the reaction of the raw materials is complete, filter to remove excess Zinc powder, concentrated to remove acetic acid, added ethyl acetate 40mL, washed with saturated sodium bicarbonate solution, concentrated organic phase, and purified by column chromatography to obtain compound 22 (315mg, yield 26.1%), LC-MS (APCI):m / z=332(M+1) + .
[0159] Step 2: Synthesis of Compound 23.
[0160] Add compound 22 (252mg, 0.76mmol) to the reaction flask, add lithium hydroxide (159mg, 3.8mmol), add water (1mL), tetrahydrofuran (5mL), and react at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com