Gene detection kit and method for screening SMA genetic diseases
A genetic disease and kit technology, applied in the field of genetic disease genetic screening, can solve the problems of inability to distinguish heterozygotes, easily cause pollution, cumbersome process, etc., achieve reliable detection results, accurate detection results, and avoid false negative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The present invention first proposes a primer and probe for fluorescent quantitative detection of SMA genetic disease, including a specific primer pair for amplifying SMN1Exon7, a specific probe for SMN1Exon7, a specific primer pair for amplifying SMN1Exon8, a specific probe for SMN1Exon8, The internal reference ALB specific primer pair of SMN1Exon7, the internal reference ALB specific probe of SMN1Exon7, the internal reference ALB specific primer pair of SMN1Exon8, the internal reference ALB specific probe of SMN1Exon8; wherein the upstream primer and downstream of the specific primer pair of SMN1Exon7 are amplified The primer sequence is shown in SEQ ID NO:1 and SEQ ID NO:2, the SMN1Exon7 specific probe sequence is shown in SEQ ID NO:3, and the upstream primer and downstream primer sequence of the specific primer pair for amplifying SMN1Exon8 are shown in SEQ ID Shown in NO:4 and SEQ ID NO:5, the SMN1Exon8-specific probe sequence is as SEQID NO:6; the upstream primer a...
Embodiment 2
[0068] Embodiment 2: Using the kit and method of the embodiment to screen clinical blood samples
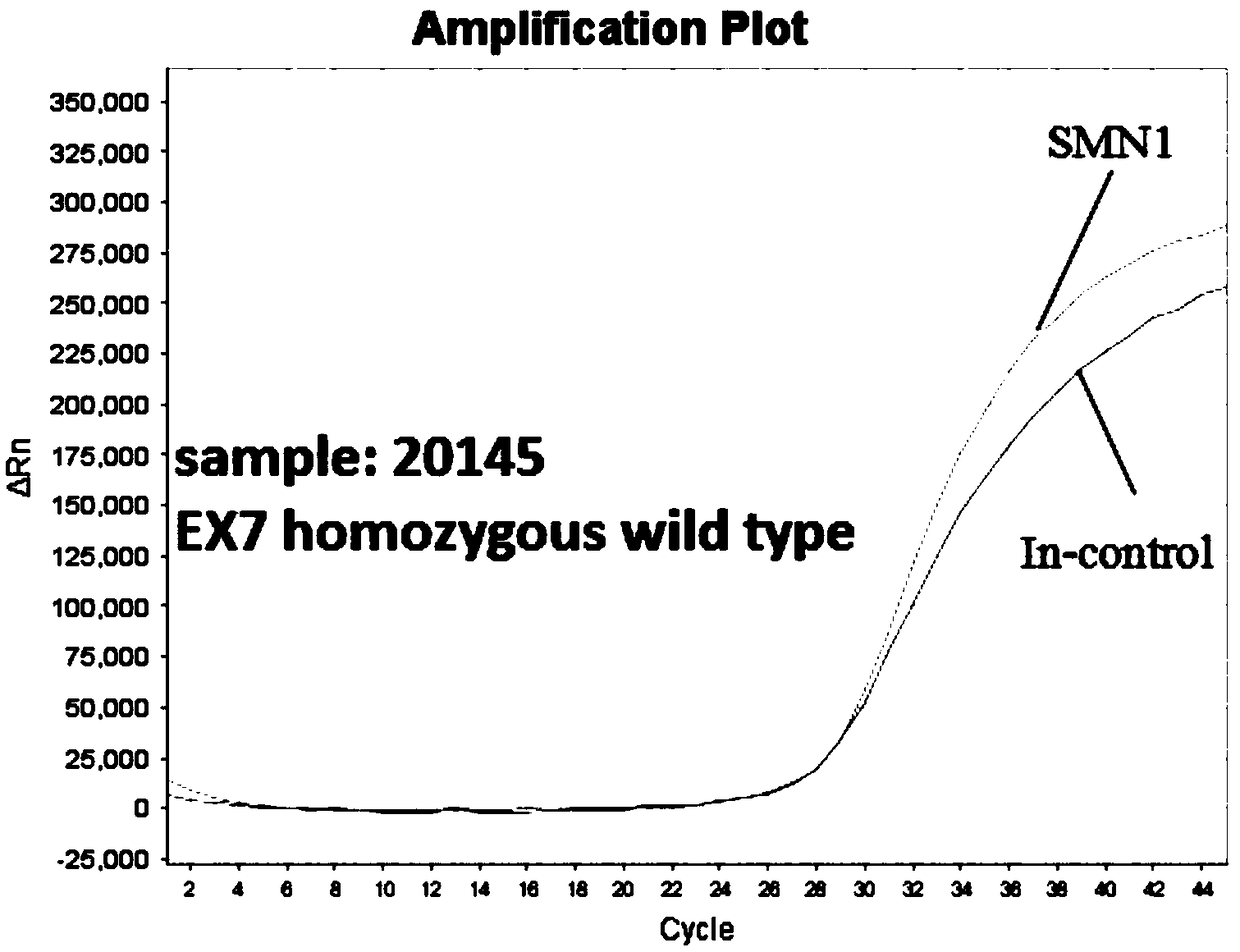
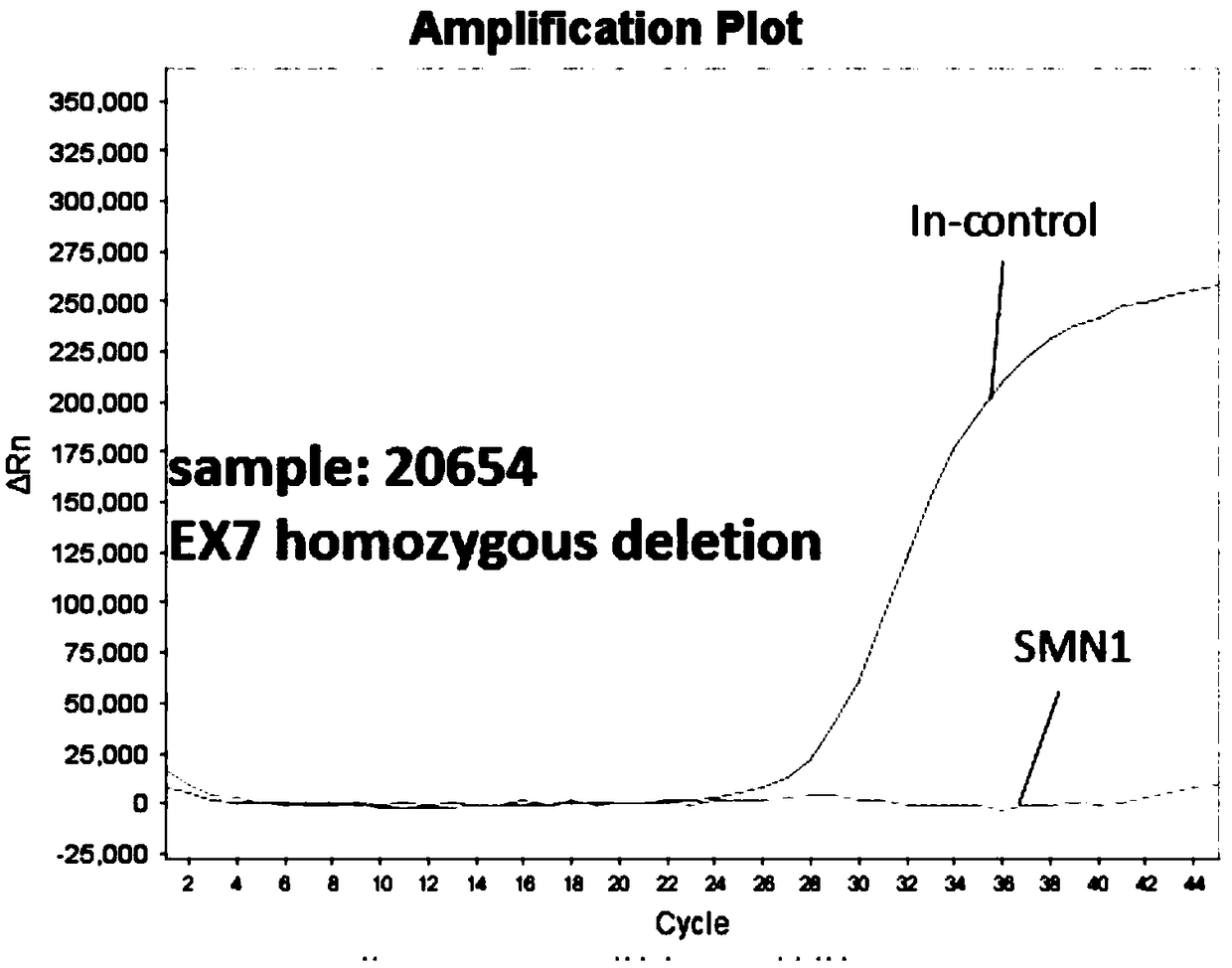
[0069]1. Select 3 clinical blood samples with known clinical results, and the sample numbers are sample 20145, sample 20491, and sample 20654.
[0070] 2. Use Tiangen Blood DNA Extraction Kit to extract DNA from the above three clinical samples.
[0071] 3. Using nanodrop2000 to measure the DNA concentration and purity of the above three samples, the results are shown in Table 1:
[0072] Table 1
[0073] sample number
[0074] 4. Dilute the DNA sample with known concentration in step 3 to 5ng / μL for later use
[0075] Table 2
[0076] sample number
[0077] 5. Take out the prepared two kinds of detection solution premix Mix (2×) from the -20°C refrigerator, let it dissolve at room temperature, wait until it is completely dissolved, mix it upside down and centrifuge slightly, and prepare 8 reaction mixes (4 wells each Dispense 23 μl of reaction solution ⅠM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com