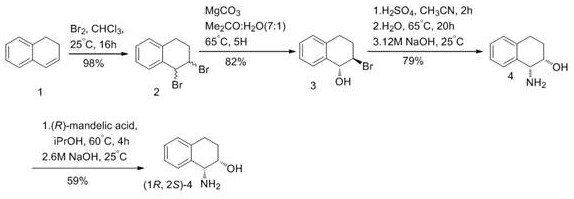
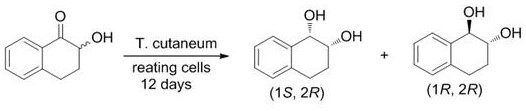
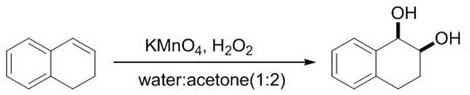
A method for efficiently synthesizing (1r,2s)-1,2-dihydronaphthalene derivatives
A technology for dihydronaphthalene derivatives and compounds, which is applied in the fields of chemical synthesis and chemistry, can solve the problems of harsh reaction conditions, inability to prepare cis-diol compounds, and long reaction time, and achieves easy reaction, low cost and high efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Tert -butyl((1 R ,2 S )-2-(((( E )-benzylidene)amino)oxy)-1,2-dihydronaphthalen-1-yl)carbamate was synthesized: 2.3mgPd(AcO) 2 and 8.2mg ( R )-Difluorphos was stirred in 1mL 1,2-dichloroethane for 30min, followed by the addition of 7.3mgZn(OTf) 2Stir for 10 min, then add 50 mg 4 Å molecular sieves, 48.6 mg of azabenzonorbornene protected by Boc and 1 ml of 1,2-dichloroethane and stir for 10 min, then add 72.6 mg of benzaldoxime, then plug the stopper and stop the reaction The glove box was taken out, and the reaction was stirred in an oil bath at 90°C. After the reaction was monitored by TLC, a white solid was obtained by silica gel column chromatography after concentration, with a yield of 78% and an ee value of 94%.
[0043] White solid, 78% yield, 94% ee .mp 126-128 o C. [α] D 20 = +358.3 (c =0.40, CH 2 Cl 2 ). 1 H NMR (400 MHz, CDCl 3 ): δ 7.99 (s, 1H), 7.48-7.45 (m,2H),7.39-7.24 (m, 6H), 7.16-7.14 (m, 1H), 6.68 (d, J =10.0 Hz, 1H), 6.21 (dd, J =4.0...
Embodiment 2
[0045] Tert -butyl((1 R ,2 S )-2-(((( E )-4-methylbenzylidene)amino)oxy)-1,2-dihydronaphthalen-1-yl)carbamate was synthesized: 2.3mgPd(AcO) 2 and 8.2mg ( R )-Difluorphos was stirred in 1mL 1,2-dichloroethane for 30min, followed by the addition of 7.3mgZn(OTf) 2 Stir for 10min, then add 50mg of 4 Å molecular sieves, 48.6mg of azabenzonorbornene protected by Boc and 1ml of 1,2-dichloroethane and stir for 10min, then add 81.1mg of p-tolualdehyde oxime, then stopper The stopper took the reaction out of the glove box, placed the reaction in an oil bath at 90°C and stirred it. After the reaction was monitored by TLC, it was concentrated and subjected to silica gel column chromatography to obtain a white solid with a yield of 55% and an ee value of 94%.
[0046] White solid, 55% yield, 94% ee .mp 103-105 o C. [α] D 20 = +332.8 (c =0.86, CH 2 Cl 2 ). 1 H NMR (400 MHz, CDCl 3 ): δ 8.00 (s, 1H), 7.40 (t, J =8.0 Hz,3H),7.30-7.26 (m, 2H), 7.17 (t, J =4.0 Hz, 3H), 6.71 (d,...
Embodiment 3
[0048] Tert -butyl((1 R ,2 S )-2-(((( E )-3-methylbenzylidene)amino)oxy)-1,2-dihydronaphthalen-1-yl)carbamate synthesis: 2.3mg Pd(AcO) 2 and 8.2mg ( R )-Difluorphos was stirred in 1mL 1,2-dichloroethane for 30min, followed by the addition of 7.3mg Zn(OTf) 2 Stir for 10min, then add 50mg 4 Å molecular sieves, 48.6mg azabenzonorbornene protected by Boc and 1ml 1,2-dichloroethane and stir for 10min, then add 81.1mg m-tolualdehyde oxime, then stopper The stopper took the reaction out of the glove box, and placed the reaction in an oil bath at 90°C to stir. After the reaction was monitored by TLC, it was concentrated and passed through a silica gel column to obtain a white solid with a yield of 72% and an ee value of 94%.
[0049] White solid, 79% yield, 94% ee .mp 74-76 o C. [α] D 20 = +366.7 (c = 0.32,CH 2 Cl 2 ). 1 H NMR (400 MHz, CDCl 3 ): δ 8.30 (s, 1H), 7.58 (d, J =7.6 Hz,1H), 7.42(d, J =5.2 Hz, 1H), 7.33-7.25 (m, 3H), 7.18 (dd, J =7.2Hz, J =10.8 Hz, 3H), 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com