Negative type photosensitive composition curable at low temperature
A photosensitive composition and technology of the composition are applied in the direction of photosensitive material processing, optics, opto-mechanical equipment, etc., to achieve the effect of excellent electrical insulation properties and cheap manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0182]
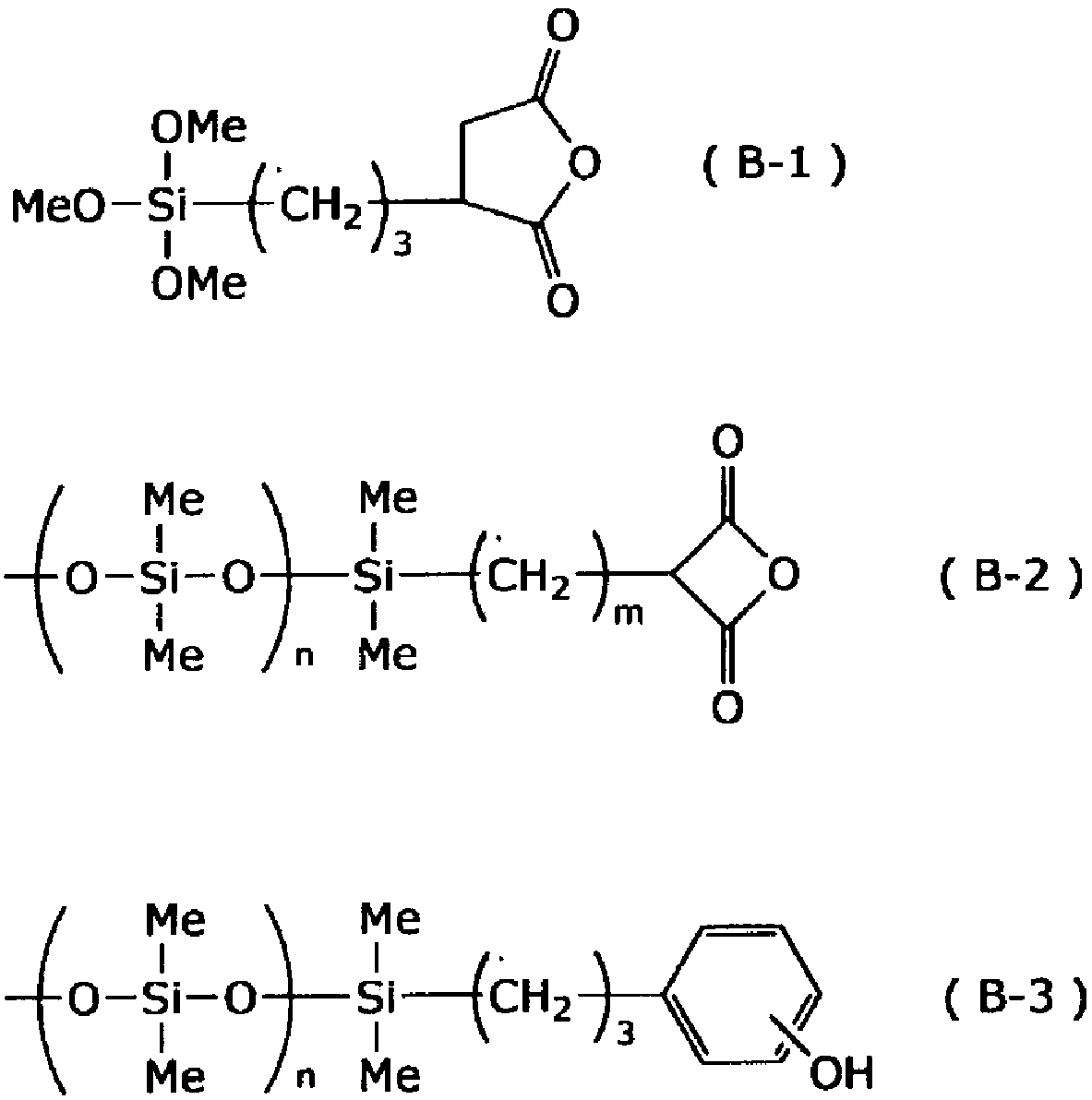
[0183] Into a reaction container equipped with a stirrer, a thermometer, and a condenser, 32.5 g of a 25% by weight TMAH aqueous solution, 800 ml of isopropanol (IPA), and 2.0 g of water were added. Separately, 39.7 g of phenyltrimethoxysilane, 34.1 g of methyltrimethoxysilane, and 7.6 g of tetramethoxysilane were mixed to prepare a mixed solution, which was then placed in a dropping funnel. This mixed solution was added dropwise into the aforementioned reaction vessel at 10°C, and after stirring the obtained mixture at the same temperature for 3 hours, 10% HCl aqueous solution was added to neutralize the mixture, and then 400 ml of toluene was added to the neutralized mixture. The mixture was separated into two layers with 100 ml of water, the organic layer was collected, concentrated under reduced pressure to remove the solvent, and PGMEA was added to the concentrate so that the solid content concentration became 40% by weight.
[0184] When the molecular weight (...
Synthetic example 2
[0185]
[0186] Add the solvent shown in Table 2 to a reaction vessel equipped with a stirrer, a thermometer, a condenser, and a nitrogen inlet pipe, heat under a nitrogen atmosphere, and heat up to an appropriate temperature with the 10-hour half-life temperature of the initiator as a reference. . Separately, the monomers shown in Table 1 were mixed with the initiators shown in Table 2 to prepare a mixture, which was then added dropwise to the aforementioned solvent over 4 hours. Then, it reacted for 3 hours, and the resin solution of alkali-soluble resin A-H was prepared. The compounding quantity in a table|surface is shown by weight part.
[0187] Table 1
[0188]
[0189] Note:
[0190] AA: Acrylic,
[0191] MAA: methacrylic acid,
[0192] KBM-503 (trade name, manufactured by Shin-Etsu Chemical Co., Ltd.): γ-methacryloxypropyltrimethoxysilane,
[0193] KBM-502 (trade name, manufactured by Shin-Etsu Chemical Co., Ltd.): γ-methacryloxypropylmethyldimethoxysilane,
...
Embodiment 1
[0210] The solution of polysiloxane obtained in Synthesis Example 1 and the solution of alkali-soluble resin A and A' obtained in Synthesis Example 2 were carried out in a weight ratio of 3:3.5:3.5 in terms of solid content of the resin. mixed to obtain a polymer mixture. This polymer mixture was mixed with acrylic monomer A (tris(2-acryloyloxyethyl)isocyanurate), acrylic monomer B and acrylic monomer C in an amount of 10 parts by weight each. These monomers are represented by the following general formula and serve as (meth)acryloyloxy group-containing compounds. Then, add 3.0 parts by weight of Irgacure OXE-02 (radical generator A) as a photoradical generator and 0.3 parts by weight of KF-53 (trade name, manufactured by Shin-Etsu Chemical Co., Ltd.) as a surfactant, and then add PGMEA made the concentration 35%, obtained the dissolution rate of Compositions. Here, the compounding ratio (parts by weight) of each component is based on 100 parts by weight of the total weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com