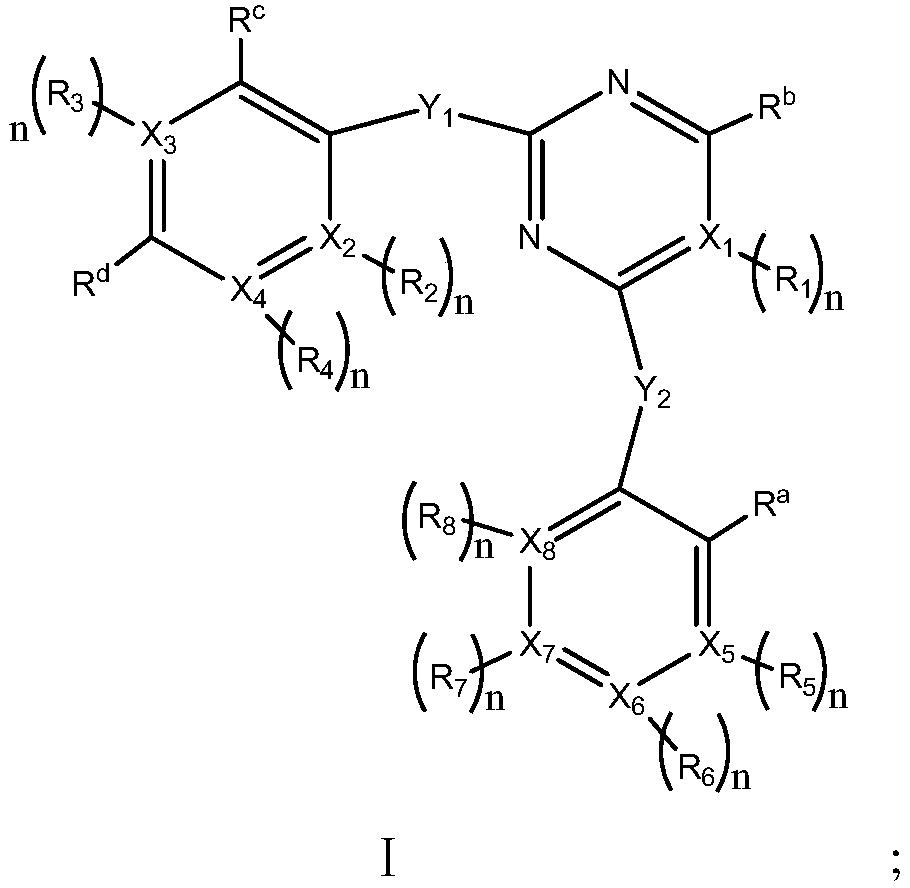
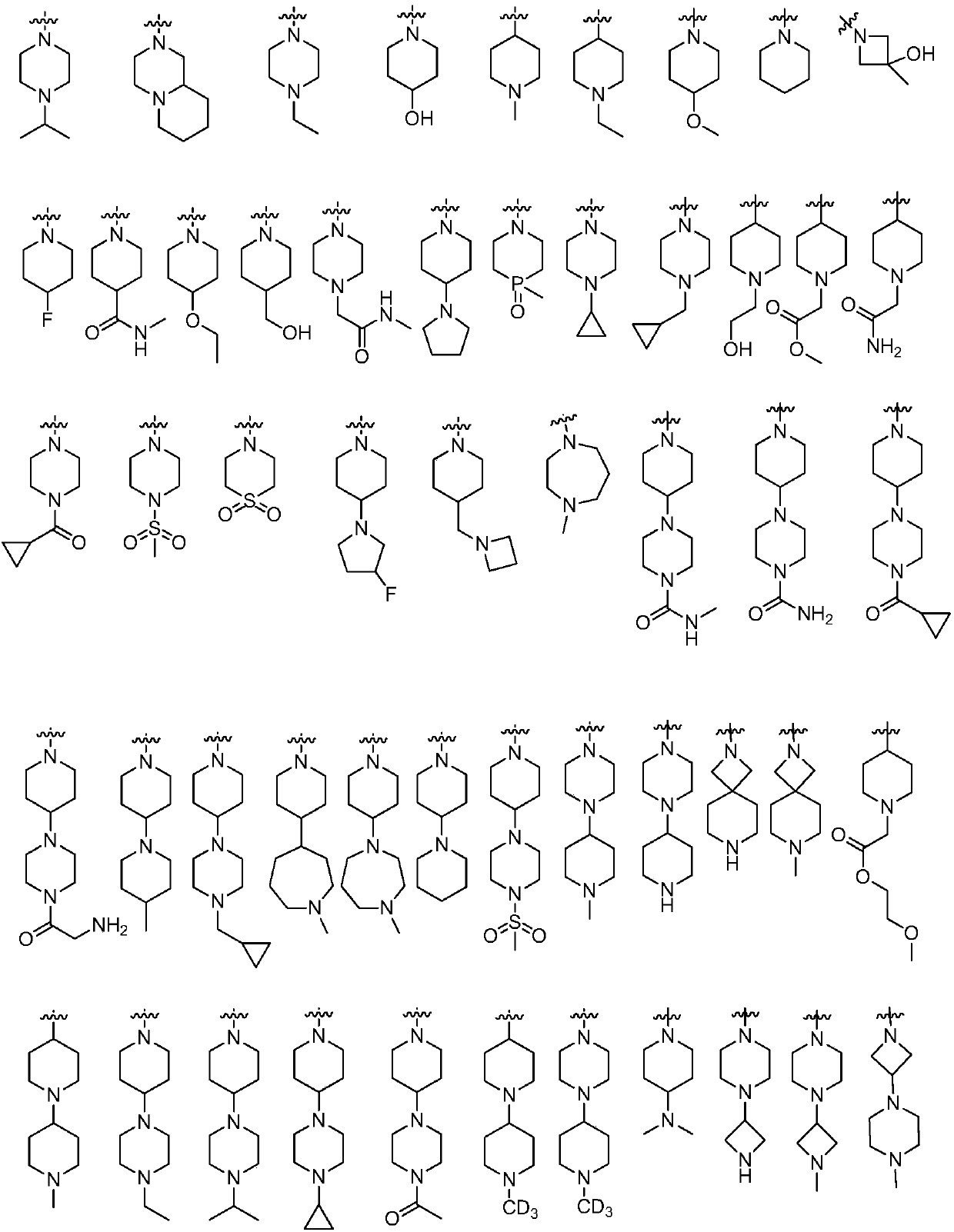
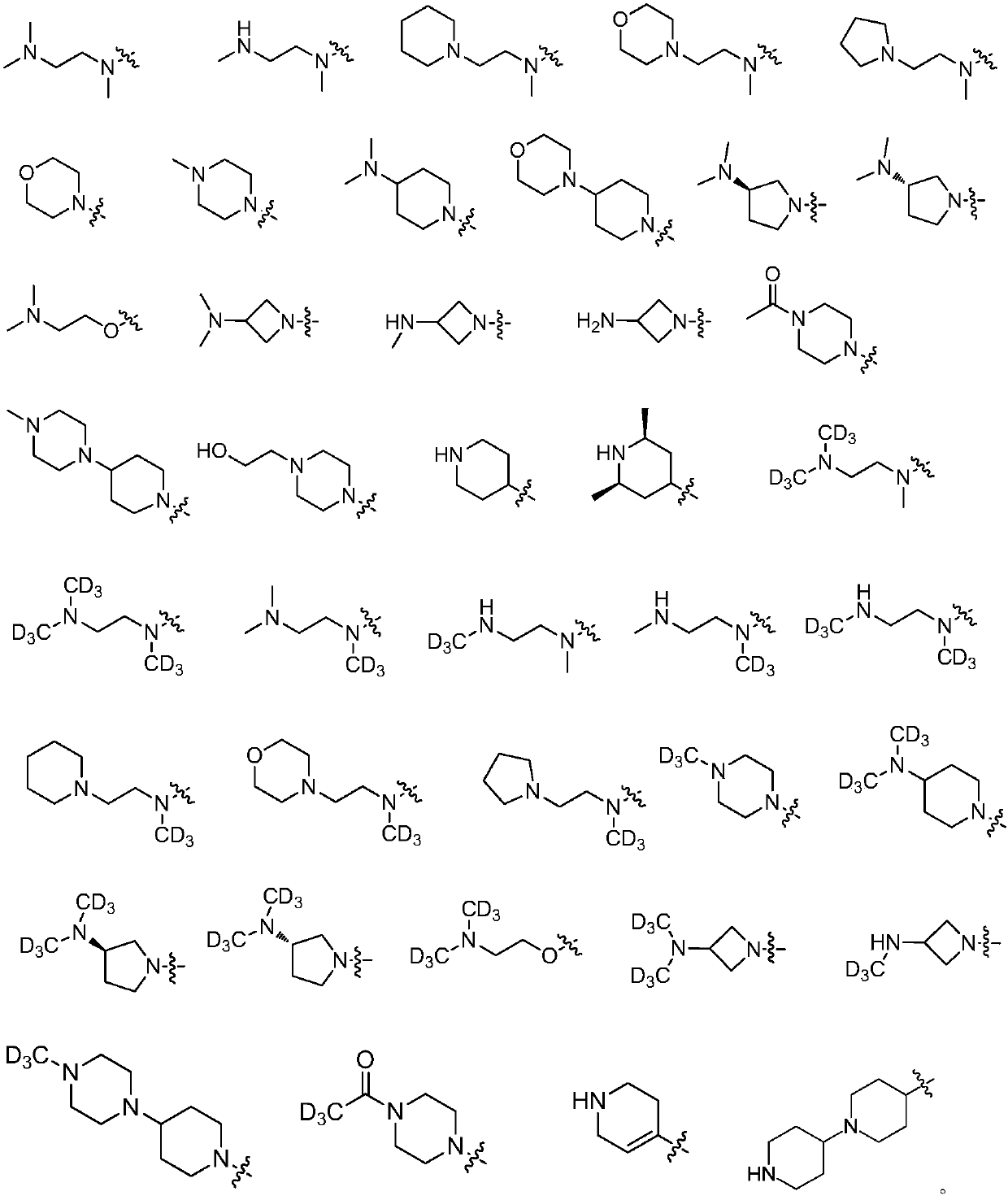
Compound used as ALK (anaplastic lymphoma kinase) inhibitor and application thereof
A compound and solvate technology, applied in the field of medicine, can solve problems such as visual impairment, elevated liver transaminase levels, and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] The synthesis of compound c-1, reaction formula is as follows:
[0110]
[0111] 1) Synthesis of compound b3-1: Put compound b1-1 (0.5g, 3mmol) into a 100mL three-necked flask, add DMF (30mL), then add compound b2 (0.86g, 4.7mmol), anhydrous potassium carbonate ( 1.23g, 9.5mmol), stirred overnight at 60°C, TLC showed that the reaction was basically complete, cooled to room temperature, added 100mL of water, extracted with ethyl acetate, washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, concentrated through the column, and obtained 0.7g khaki solid, 1 H NMR (400MHz, DMSO): δ11.82(s, 1H), 8.45(m, 2H), 7.68(m, 2H), 7.27(m, 1H), 1.79(d, J=13.6Hz, 6H); MS m / z(ESI): 316[M+H] + .
[0112] 2) Synthesis of compound c-1: Dissolve compound a6 (150 mg, 0.5 mmol) in acetone / water (volume ratio 3:1, 8 mL in total), add compound b3-1 (200 mg, 0.6 mmol), 3 drops of concentrated hydrochloric acid , sealed, heated to 100°C, stirred overnight, c...
Embodiment 2
[0114] The synthesis of compound c-2, reaction formula is as follows:
[0115]
[0116] 1) Synthesis of compound b3-2: Put compound b1-2 (2g, 16.3mmol) into a 100mL three-necked flask, add DMF (70mL), then add compound b2 (4.65g, 25mmol), anhydrous potassium carbonate (7.16 g, 52mmol), stirred overnight at 60°C, TLC showed that the reaction was basically complete, cooled to room temperature, added 100mL of water, extracted with ethyl acetate, washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, concentrated through the column to obtain 1.5g yellow solid, 1 H NMR (400MHz, CDCl 3 ): δ8.51(d,J=8Hz,1H),8.18(s,1H),8.13(br,,1H),7.07(m,2H),6.95(d,J=8Hz 1H); MS m / z(ESI): 270[M+H] + .
[0117] 2) Synthesis of compound c-2: Dissolve compound a6 (150 mg, 0.5 mmol) in acetone / water (3:1, 8 mL), add compound b3-2 (190 mg, 0.6 mmol) and 3 drops of concentrated hydrochloric acid, seal and heat to 100°C, stirred overnight, cooled to room temperature,...
Embodiment 3
[0119] The synthesis of compound c-3, reaction formula is as follows:
[0120]
[0121] 1) Synthesis of compound b3-3: Put compound b1-3 (2g, 16.3mmol) into a 100mL three-necked flask, add DMF (70mL), then add compound b2 (4.65g, 25mmol), anhydrous potassium carbonate (7.16 g, 52mmol), stirred overnight at 60°C, TLC showed that the reaction was basically complete, cooled to room temperature, added 100mL of water, extracted with ethyl acetate, washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, concentrated through the column to obtain 1.5g yellow solid, 1 H NMR (400MHz, CDCl 3 ): δ8.19(s,1H),7.63(d,J=8.4Hz,2H),7.63(t,J=7.6Hz,2H),7.21(s,1H),7.17(t,J=7.2Hz ,2H); MS m / z (ESI): 240[M+H] + .
[0122] 2) Synthesis of compound c-3: Dissolve compound a6 (150 mg, 0.5 mmol) in acetone / water (3:1, 8 mL), add compound b3-3 (190 mg, 0.6 mmol) and 3 drops of concentrated hydrochloric acid, seal and heat to 100°C, stirred overnight, cooled to room t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com