Four astraisoflavan-7-O-beta-D-glucoside compounds with nerve cell protection activity and preparation method thereof
A nerve cell protection and isoflavane glycoside technology, which is applied in the field of four isoflavane glycoside compounds and their preparation, can solve the problems of unseen compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
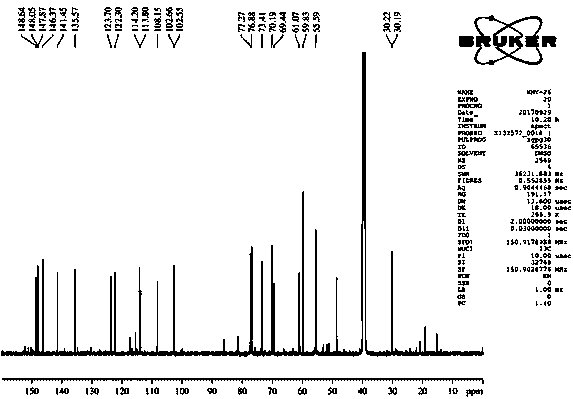
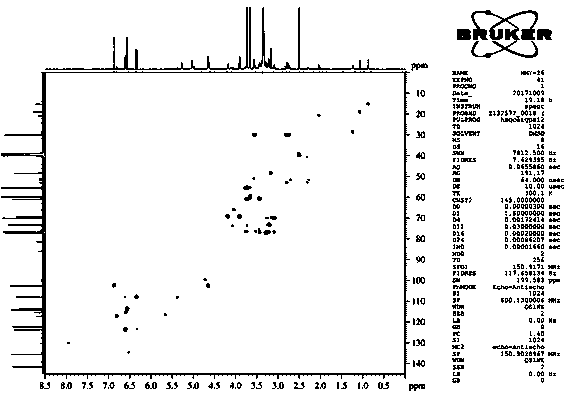
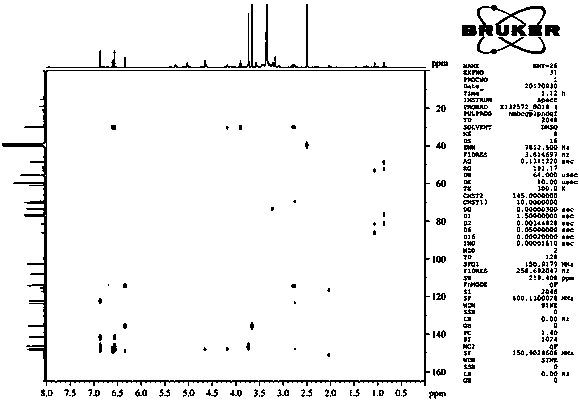
[0046] Isolation and purification of compound 1-4:
[0047] Medicago sativa ( Medicago sativa L.) roots 7 kg, 70% ethanol was heated and refluxed for extraction three times, each for 2 hours. The extract was concentrated under reduced pressure to obtain the total extract. The total extract was suspended and dispersed in water, extracted three times with dichloromethane, and concentrated under reduced pressure to obtain 1.5 kg of aqueous layer extract. Separate 1.5 kg of the aqueous layer extract with macroporous resin D101, perform gradient elution according to methanol:water 0:100, 30:70, 50:50, 70:30, 100:0, and concentrate under reduced pressure to obtain fraction Fr . AE five stream points. The component Fr. D was subjected to normal phase silica gel column chromatography, and the ratio of dichloromethane: methanol was 50:1, 30:1, 15:1, 8:1, 4:1, 2:1, 1:1, 0:1 gradient elution was carried out, and 8 fractions (Fr. D1-D8) were obtained, of which D3 was subjected to silica g...
Embodiment 2
[0060] The pharmacological effects of the compound of the present invention and the pharmaceutical composition composed of pharmaceutical excipients in neurodegenerative diseases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com