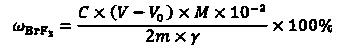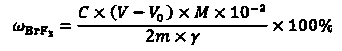Detection method of purity of bromine trifluoride product
A bromine trifluoride, product purity technology, applied in chemical method analysis, preparation of test samples, material analysis by observing the impact on chemical indicators, etc., can solve the problem that there is no unified standard for bromine trifluoride purity detection. , There are no enterprise standards, industry standards or other standards, etc., to achieve the effect of high safety, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A detection method for bromine trifluoride product purity, comprising the following steps:
[0046] (I) Pretreatment of products
[0047] (i) Pour 2.3747 g of bromine trifluoride from the sample bottle into the PTFE sample tube; put the PTFE sample tube containing the sample and the 500 mL PTFE hydrolysis bottle containing 40 mL of deionized water into the refrigerator Freezing treatment in
[0048] (ii) After the sample in the PTFE sample tube and the water in the PTFE hydrolysis bottle are solidified, unscrew the cap of the PTFE hydrolysis bottle and the cap of the PTFE sample tube, and throw the PTFE sample tube into the In the PTFE hydrolysis bottle, quickly tighten the cap of the hydrolysis bottle, and wait for it to melt and react;
[0049] (iii) Transfer the solution obtained in step (ii) to a volumetric flask, and dilute to 250 mL with deionized water to obtain the sample hydrolyzate;
[0050] (II) Extraction of product hydrolyzate
[0051] Take 100 mL of th...
Embodiment 2
[0055] A detection method for bromine trifluoride product purity, comprising the following steps:
[0056] (I) Pretreatment of products
[0057] (i) Pour 1.8243 g of bromine trifluoride from the sample bottle into the PTFE sample tube; put the PTFE sample tube containing the sample and the 500 mL PTFE hydrolysis bottle containing 40 mL of deionized water into the refrigerator Freezing treatment in
[0058] (ii) After the sample in the PTFE sample tube and the water in the PTFE hydrolysis bottle are solidified, unscrew the cap of the PTFE hydrolysis bottle and the cap of the PTFE sample tube, and throw the PTFE sample tube into the In the PTFE hydrolysis bottle, quickly tighten the cap of the hydrolysis bottle, and wait for it to melt and react;
[0059] (iii) Transfer the solution obtained in step (ii) to a volumetric flask, and dilute to 250 mL with deionized water to obtain the sample hydrolyzate;
[0060] (II) Extraction of product hydrolyzate
[0061] Take 100 mL of th...
Embodiment 3
[0065] A detection method for bromine trifluoride product purity, comprising the following steps:
[0066] (I) Pretreatment of products
[0067] (i) Pour 1.7397 g of bromine trifluoride from the sample bottle into the PTFE sample tube; put the PTFE sample tube containing the sample and the 500 mL PTFE hydrolysis bottle containing 40 mL of deionized water into the refrigerator Freezing treatment in
[0068] (ii) After the sample in the PTFE sample tube and the water in the PTFE hydrolysis bottle are solidified, unscrew the cap of the PTFE hydrolysis bottle and the cap of the PTFE sample tube, and throw the PTFE sample tube into the In the PTFE hydrolysis bottle, quickly tighten the cap of the hydrolysis bottle, and wait for it to melt and react;
[0069] (iii) Transfer the solution obtained in step (ii) to a volumetric flask, and dilute to 250 mL with deionized water to obtain the sample hydrolyzate;
[0070] (II) Extraction of product hydrolyzate
[0071] Take 100 ml of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com