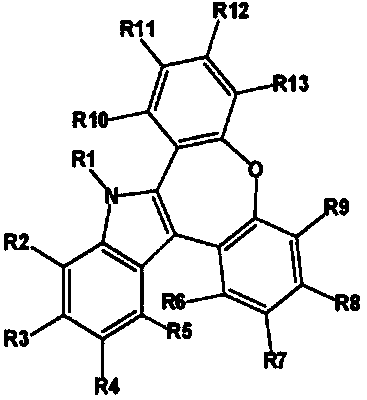
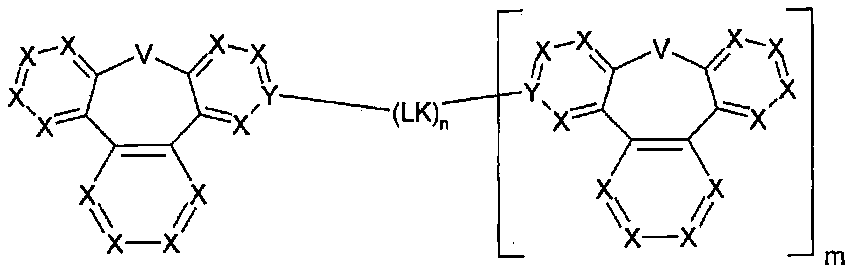
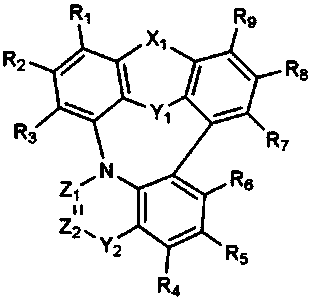
Organic electroluminescent compound and organic electroluminescent device comprising the same
An electroluminescent device and electroluminescent technology, applied in electroluminescent light sources, organic chemistry, electric light sources, etc., can solve the problems of no advantage in power efficiency, low glass transition temperature, shortened device life, etc., and achieve excellent power efficiency , The effect of low driving voltage and improving driving life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Embodiment 1: preparation compound C-39
[0115]
[0116] Preparation of compound 1-1
[0117] 2-Bromo-1-chloro-nitrobenzene (56g, 234mmol), 2-chlorophenylboronic acid (74g, 476mmol), tetrakis (triphenylphosphine) palladium (0) (Pd (PPh 3 ) 4 ) (13g, 11.9mmol), 2M cesium carbonate (194g, 596mmol), toluene (1200mL) and ethanol (300mL) were poured into a flask and dissolved, then refluxed for 12 hours. After the reaction was completed, the organic layer was extracted with ethyl acetate. The organic extract was dried over magnesium sulfate, and purified by column chromatography to obtain compound 1-1 (51 g, yield: 80%).
[0118] Preparation of Compound 1-2
[0119] Compound 1-1 (10g, 37.3mmol), 2-methoxyphenylboronic acid (6.8g, 44.7mmol), tris(dibenzylideneacetone) dipalladium (1.7g, 1.86mmol), cesium carbonate (30g , 93.2mmol), tricyclohexylphosphine (1g, 3.73mmol), toluene (200mL) and dioxane (50mL) were poured into the flask and dissolved, then refluxed fo...
Embodiment 2
[0129] Embodiment 2: preparation compound C-36
[0130]
[0131] Compound 1-5 (5g, 19mmol), 2-(3-bromobiphenyl)-3-yl-4,6-diphenyl-1,3,5-triazine (9g, 19mmol), palladium acetate ( 0.2g, 0.97mmol), sodium tert-butoxide (4.6g, 48mmol), 2-dichlorohexylphosphine-2',6'-dimethoxybiphenyl (0.7g, 1.9mmol) and o-xylene (100mL ) into the flask, dissolved, and then refluxed for 7 hours. After the reaction was completed, the organic layer was extracted with ethyl acetate. The organic extract was dried over magnesium sulfate, and purified by column chromatography to obtain compound C-36 (8.2 g, yield: 65%).
[0132] compound
Embodiment 3
[0133] Embodiment 3: preparation compound C-31
[0134]
[0135] Compound 1-5 (3g, 11mmol), 2-[1,1'-biphenyl]-4-yl-4-chloro-6-phenyl-1,3,5-triazine (4.8g, 14mmol) , 4-(dimethylamino)pyridine (0.7g, 5.8mmol), potassium carbonate (4g, 29mmol) and dimethylformamide (120mL) were poured into a flask, dissolved, and heated to 120°C, and then reacted for 4 Hour. After completing the reaction, the mixture was added dropwise to distilled water, and the resulting solid was filtered. The filtrate was dried and purified by column chromatography to obtain compound C-31 (6.4 g, yield: 97%).
[0136] compound
PUM
| Property | Measurement | Unit |
|---|---|---|
| process yield | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
| process yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com