Preparation method of plasma quality control product for quality control of centrifugal microfluidic chip
A microfluidic chip and centrifugal technology, applied in the field of medical testing, can solve the problems of insufficient automatic biochemical testing equipment and lack of testing professionals, and achieve the effects of avoiding matrix effects, sufficient sources, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
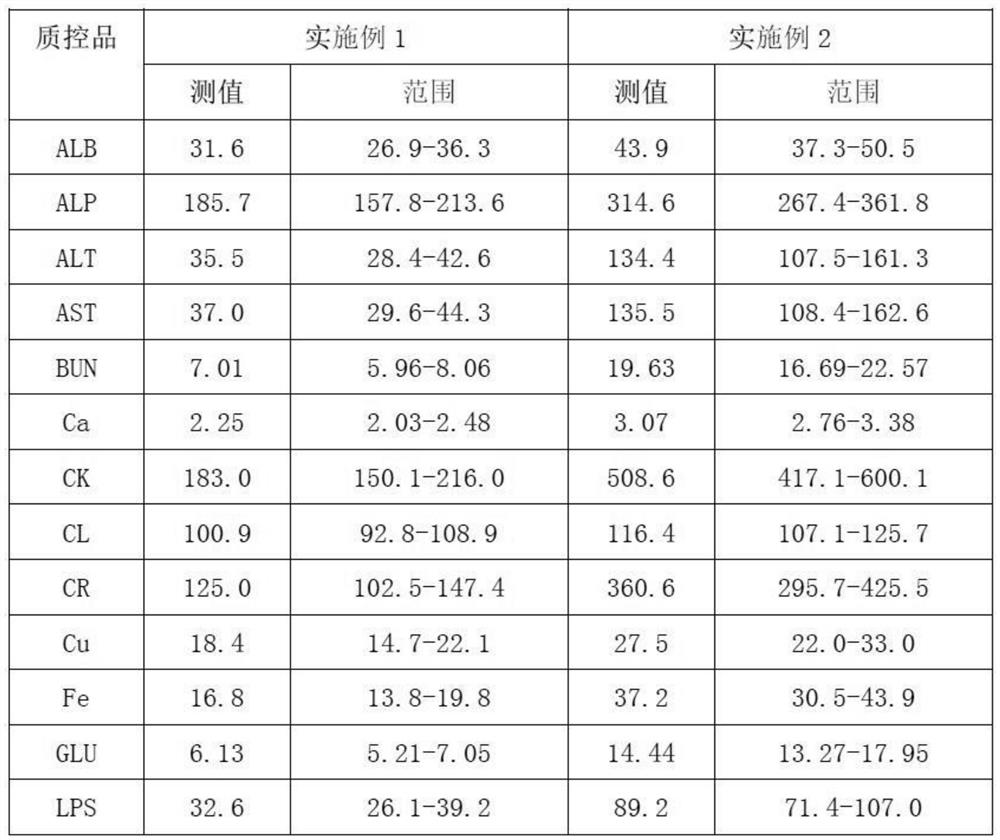
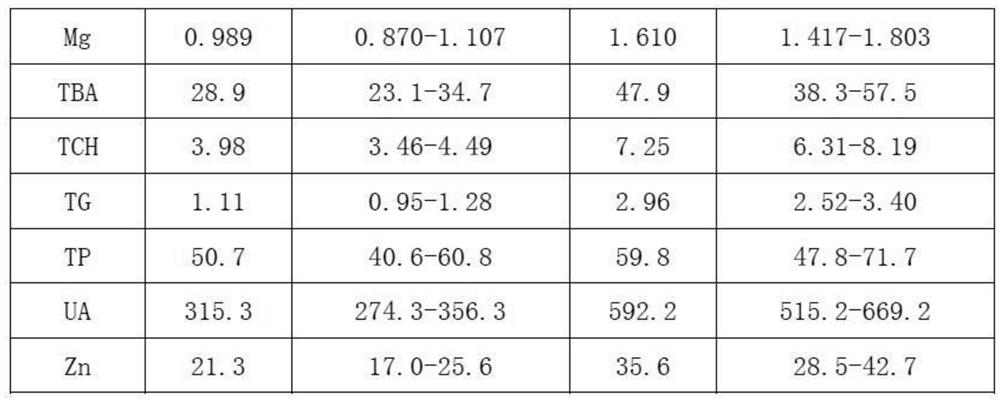
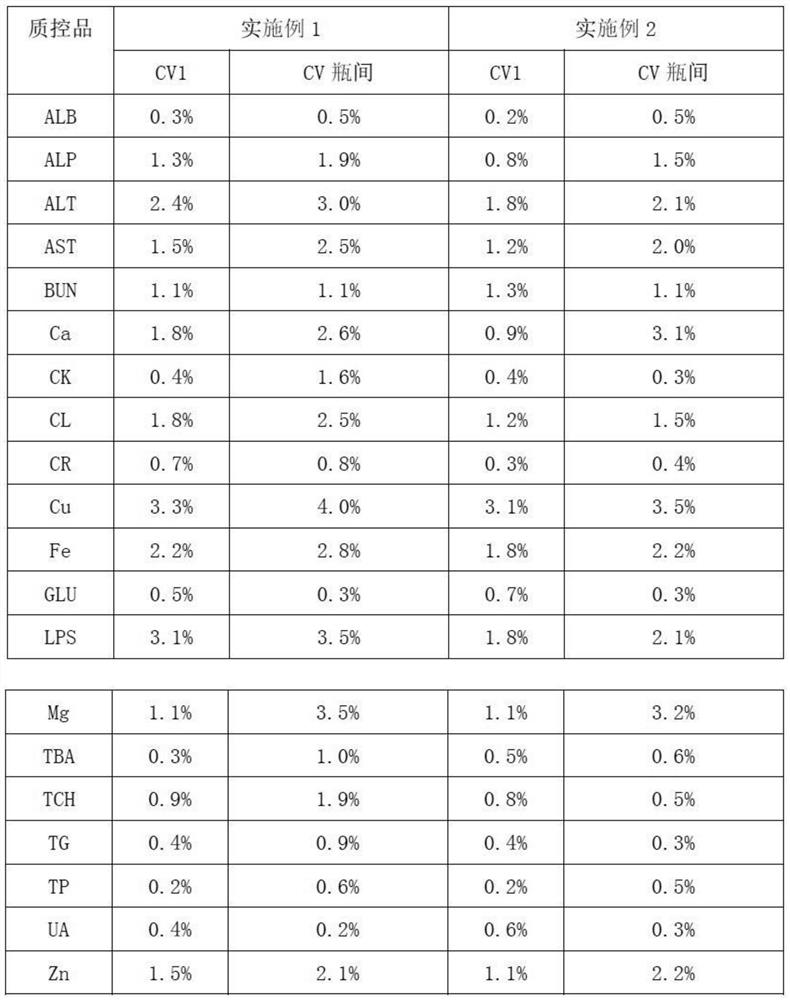
Embodiment 1
[0026] 1. Add 0.6% (mass percent) cholesterol sodium sulfate to bovine plasma, heat to 65°C, and cool to room temperature after completely dissolving;
[0027] 2. Add 0.02% (mass percentage) potassium dihydrogen phosphate to the solution obtained in step 1;
[0028] 3. Add 0.04% (mass percent) ammonium ferric sulfate dodecahydrate successively in distilled water, 0.5% (mass percent) calcium chloride, 0.2% (mass percent) magnesium sulfate heptahydrate, 0.6% (mass percent) urea (urea), 9% (mass percentage) of sodium chloride, 0.01% (mass percentage) of zinc sulfate heptahydrate, 0.01% (mass percentage) of copper sulfate pentahydrate, 0.06% (mass percentage) of glycine Cholic acid, 2% (mass percentage) of glucose, 0.03% (mass percentage) of creatinine, 0.08% (mass percentage) of uric acid, 0.07% (mass percentage) of glyceryl triolein, the dissolution of the previous material is completed and mixed uniformly Then add the next substance;
[0029] 4. Mix the solutions obtained in ...
Embodiment 2
[0033] 1. Add 1.0% (mass percent) cholesterol sodium sulfate to bovine plasma, heat to 65°C, and cool to room temperature after completely dissolving;
[0034] 2. Add 0.06% (mass percentage) potassium dihydrogen phosphate to the solution obtained in step 1;
[0035]3. Add 0.08% (mass percent) ammonium ferric sulfate dodecahydrate successively in distilled water, 0.9% (mass percent) calcium chloride, 0.6% (mass percent) magnesium sulfate heptahydrate, 1% (mass percent) urea, 13% (mass percentage) of sodium chloride, 0.03% (mass percentage) of zinc sulfate heptahydrate, 0.03% (mass percentage) of copper sulfate pentahydrate, 0.2% (mass percentage) of glycocholic acid, 4% (mass percentage) of glucose, 0.06% (mass percentage) of creatinine, 0.2% (mass percentage) of uric acid, 0.11% (mass percentage) of glyceryl trioleate, the last material is dissolved and then added after mixing evenly the next substance;
[0036] 4. Mix the solutions obtained in step 2 and step 3 uniformly ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com