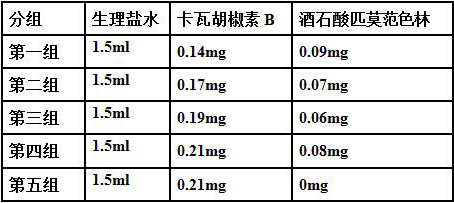
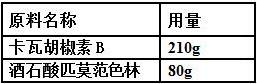
A kind of oral pharmaceutical composition for treating diabetes
A technology for diabetes and composition, which is applied in the field of oral pharmaceutical compositions for treating diabetes, can solve the problem that there is no literature report on the anti-diabetic activity of kava kava kava
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 The granule for treating diabetes and its preparation
[0027]
[0028] Preparation:
[0029] A: Take kavaperine B and pimavanserin tartrate and pass through a 100-mesh sieve, and mix well.
[0030] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0031] The amount of sugar used is 2.0 times the weight of kava B; the amount of microcrystalline cellulose is 1.2 times the weight of kava B; the amount of crospovidone is 0.12 times the weight of kava B
[0032] C: Mix the powder obtained in step A and step B evenly.
[0033] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. The dosage of povidone K30 is 0.17 times of the weight of kavaperine B; the dosage of water is 32.3 times of the weight of povidone K30.
[0034] E: The liquid dispersion system obtained in step D is added to the powder obtained in step C, granulated, dried, and the dry partic...
Embodiment 2
[0036] Example 2 Granules for treating diabetes and its preparation
[0037]
[0038] Preparation:
[0039] A: Take kavaperine B and pimavanserin tartrate and pass through a 100-mesh sieve, and mix well.
[0040] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0041] The amount of sugar used is 2.0 times the weight of kava B; the amount of microcrystalline cellulose is 1.2 times the weight of kava B; the amount of crospovidone is 0.12 times the weight of kava B
[0042] C: Mix the powder obtained in step A and step B evenly.
[0043] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. The dosage of povidone K30 is 0.17 times of the weight of kavaperine B; the dosage of water is 32.3 times of the weight of povidone K30.
[0044] E: The liquid dispersion system obtained in step D is added to the powder obtained in step C, granulated, dried, and the dry particles ar...
Embodiment 3
[0046] Embodiment 3 granule for treating diabetes and its preparation
[0047]
[0048] Preparation:
[0049] A: Take kavaperine B and pimavanserin tartrate and pass through a 100-mesh sieve, and mix well.
[0050] B: Take lactose, microcrystalline cellulose, and crospovidone to pass through a 100-mesh sieve, and mix well. which milk
[0051] The amount of sugar used is 2.0 times the weight of kava B; the amount of microcrystalline cellulose is 1.2 times the weight of kava B; the amount of crospovidone is 0.12 times the weight of kava B
[0052] C: Mix the powder obtained in step A and step B evenly.
[0053] D: Take povidone K30 and add water and stir well to make a liquid dispersion system. The dosage of povidone K30 is 0.17 times of the weight of kavaperine B; the dosage of water is 32.3 times of the weight of povidone K30.
[0054] E: The liquid dispersion system obtained in step D is added to the powder obtained in step C, granulated, dried, and the dry particles ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com