A method for staining apoptotic cells
A staining method and technology of apoptotic cells, which are applied in the preparation, sampling, and instrumentation of test samples, can solve the problems of high requirements for the staining process, expensive reagents, and damage to the tissue structure of slices, and achieve low dyeing costs and cheap reagents Easy-to-obtain, simple-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
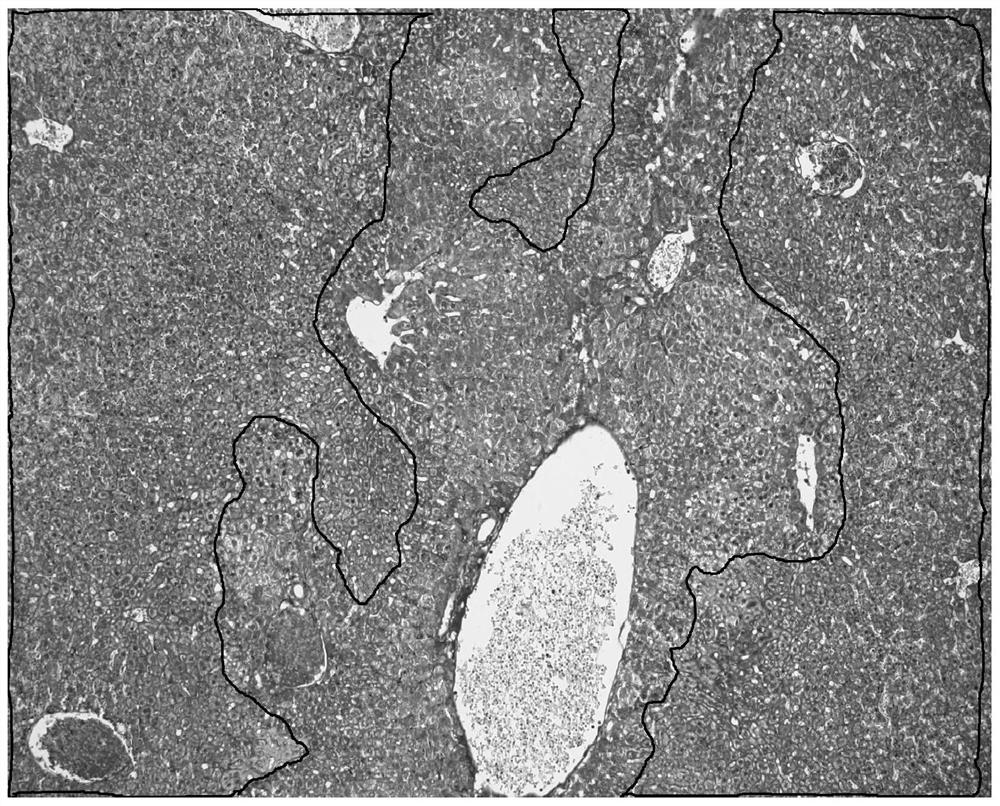
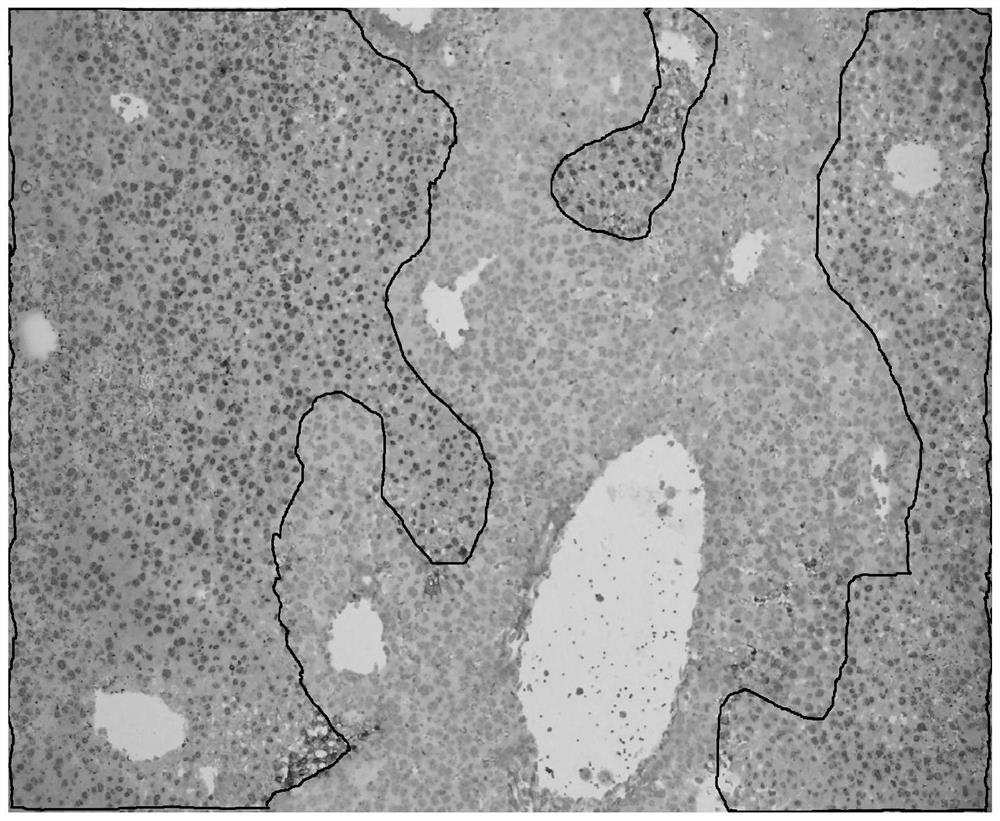
[0034] The staining object is a serial section of liver injury model after giving mice concanavalin, the staining result is as follows figure 1 As shown, using TUNEL in situ hybridization staining method as a control experiment, the control staining results are as follows figure 2 Shown. In the figure, although red blood cells are also marked with the same color as apoptotic cells, the morphology of red blood cells is significantly different from other tissue cells, and there is basically no interference in the recognition of apoptotic cells.
[0035] Follow the steps below to dye:
[0036] (1) Dewax the slices to xylene for 40 minutes, treat with absolute ethanol for 10 minutes, and then treat with 95% ethanol, 90% ethanol, 80% ethanol, 70% ethanol, 60% ethanol, 50% ethanol for 2 minutes each In, after washing with water, dye with dyeing solution I for 5 minutes;
[0037] (2) After cleaning with acetic acid aqueous solution, treat with phosphomolybdic acid aqueous solution for 8 m...
Embodiment 2
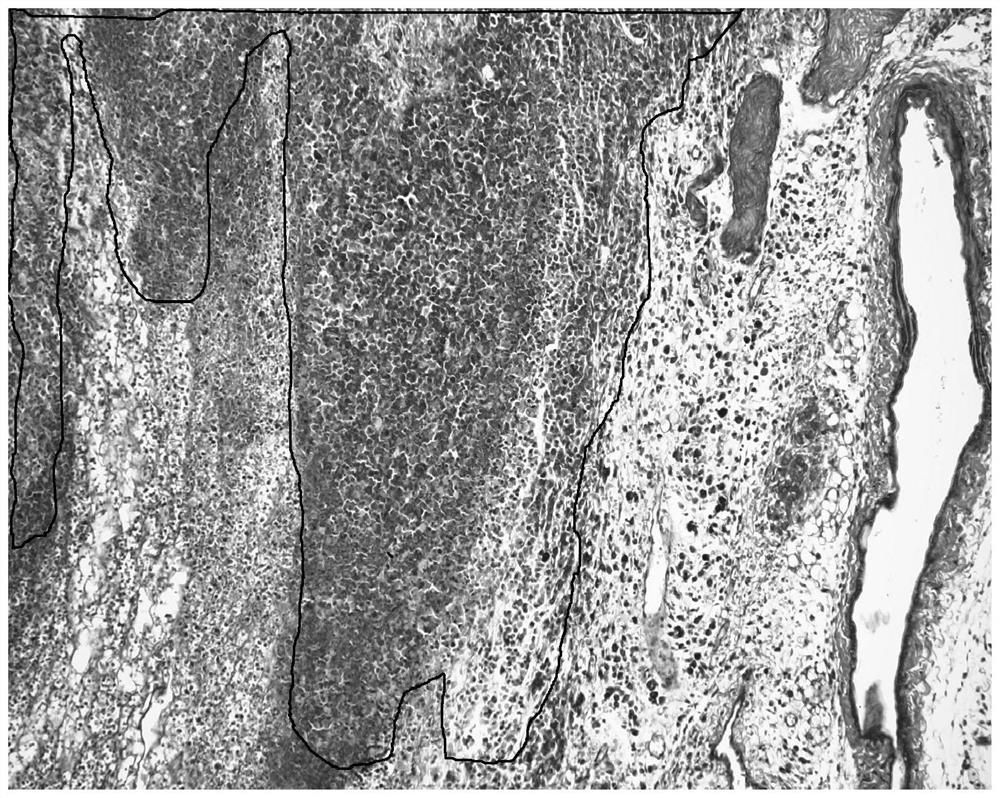
[0050] Except that the staining object is a serial section of tumor tissue grown from h22 tumor cells planted in the armpit of a mouse, the rest are the same as in Example 1. The staining results are as follows image 3 Shown. The TUNEL in situ hybridization staining method is also used as a control experiment, and the results of the control staining are as follows Figure 4 Shown.
Embodiment 3
[0052] Except for the formula of dyeing solution I:
[0053] Except for 0.1 g of acid complex red, 0.1 g of ponceau red, 0.1 g of orange G, and 300 ml of 0.2% acetic acid, the rest are the same as in Example 1.
[0054] The staining results are consistent with Example 1, which can distinguish apoptotic cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com