A kind of synthetic method of one-system 2-amino-4,6-dimethoxypyrimidine
A technique for the synthesis of dimethoxypyrimidine and its synthesis method, which is applied in the field of synthesis of one-system 2-amino-4,6-dimethoxypyrimidine, which can solve the problem of affecting product yield and quality, unfriendly process environment, and drying equipment. Complexity and other issues, to achieve the effect of saving reaction equipment, improving operating environment conditions, and reducing synthesis costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The synthetic method of 2-amino-4,6-dimethoxypyrimidine, the steps are as follows:
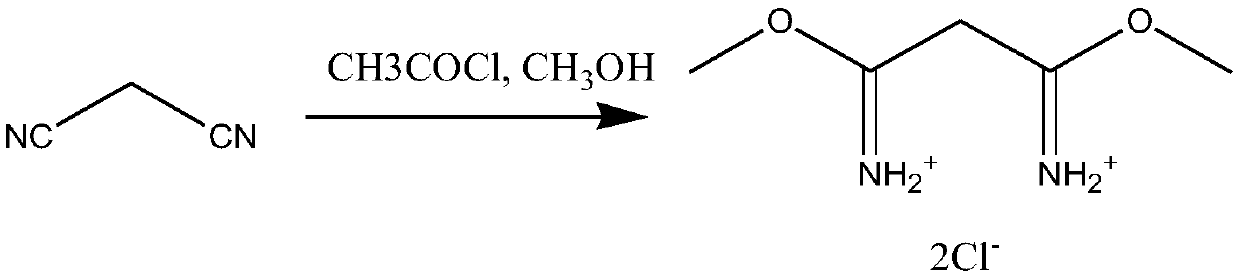
[0024] (1) Synthesis of 1,3-dimethylmalonamide dihydrochloride
[0025] Put 66g of malononitrile and 176g of anhydrous methanol into the reaction kettle, slowly drip 235.5g of acetyl chloride into the kettle, control the reaction temperature at 0-5°C, and the dripping time for 5 hours. After the dripping is completed, continue the insulation reaction 1 After hours, the hydrogen chloride in the solvent was extracted under reduced pressure to obtain 1,3-dimethylmalonamide dihydrochloride mixture.
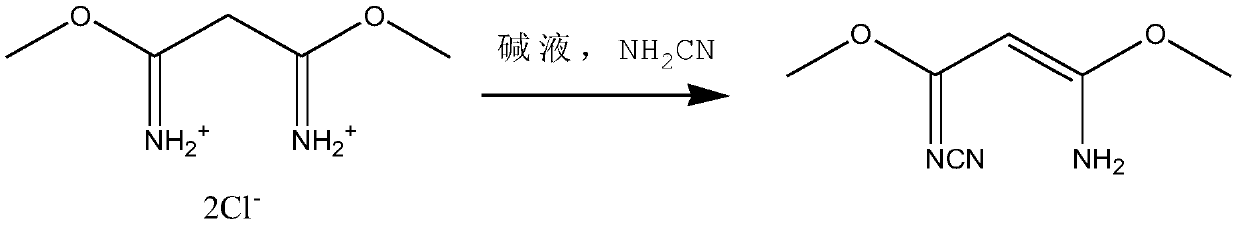
[0026] (2) Synthesis of 3-amino-3-methoxy-N-nitrile-2-propionamidine
[0027] Add lye (20g of sodium bicarbonate, 30g of sodium hydroxide, and 500g of water) and 110g of cyanamide solution with a concentration of 50wt% to the mixture of 1,3-dimethylmalamidine dihydrochloride to control the reaction temperature -5-0℃, the pH value of the reaction is 5-6, the molar ratio of the feed is that after the fe...
Embodiment 2
[0032] The synthetic method of 2-amino-4,6-dimethoxypyrimidine, the steps are as follows:
[0033] (1) Synthesis of 1,3-dimethylmalonamide dihydrochloride
[0034] Put 66g of malononitrile and 204.8g of anhydrous methanol into the reaction kettle, slowly drip 353.25g of acetyl chloride into the kettle, control the reaction temperature at 10-15°C, and the dripping time for 8 hours. After the dripping is completed, continue the insulation reaction For 2 hours, the hydrogen chloride in the solvent was extracted under reduced pressure to obtain a 1,3-dimethylmalonamide dihydrochloride mixture.
[0035] (2) Synthesis of 3-amino-3-methoxy-N-nitrile-2-propionamidine
[0036] Add lye (20g of sodium bicarbonate, 30g of sodium hydroxide, and 500g of water) and 101g of cyanamide solution with a concentration of 50wt% to the mixture of 1,3-dimethylmalamidine dihydrochloride to control the reaction temperature 0-3℃, the pH value of the reaction is 6-7, the molar ratio of the feed is that the temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com