A fluorescent probe based on pyrrole-coumarin dihydrazone derivatives and its preparation method and application
A fluorescent probe, coumarin technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as nervous system disorders, and achieve high sensitivity, easy availability of raw materials, and highly selective fluorescence recognition performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
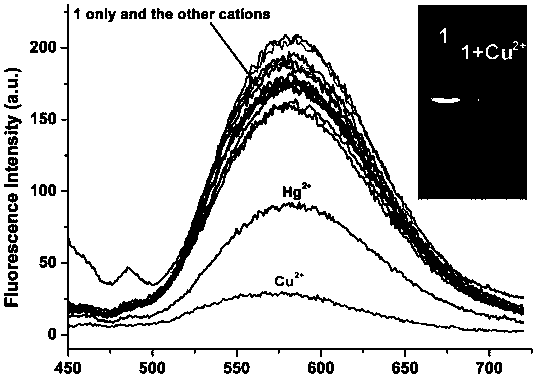
Examples
Embodiment 1
[0025] Synthesis of pyrrole-coumarin dihydrazone derivatives
[0026]
[0027] Dissolve 195mg of 3,5-dimethylpyrrole-2-carbaldehyde in 10mL of absolute ethanol, then add 218mg of 4-hydroxy-3-acetylcoumarin hydrazone, and drop in 2 drops of glacial acetic acid, reflux and stir for 3h under normal pressure After cooling to room temperature, a large amount of solids were precipitated, filtered under reduced pressure, and the filter residue was washed with absolute ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 68%.
[0028] Adopt nuclear magnetic resonance instrument to carry out nuclear magnetic resonance analysis to the pyrrole-coumarin dihydrazone derivative that makes, the result is as follows:
[0029] 1 H NMR (400MHz, DMSO-d 6 ), δ (ppm): 16.36 (s, 1H, OH), 11.22 (s, 1H, NH), 8.41 (s, 1H, CH=N), 7.96-7.98 (d, 1H, Aryl-H), 7.62 -7.66(t,1H,Aryl-H),7.27-7.34(q,1H,Aryl-H),5.83(s,1H,CH),2.93(s,3H,CH 3 ),2.19(s,3H,CH...
Embodiment 2
[0032] Dissolve 390mg of 3,5-dimethylpyrrole-2-carbaldehyde in 20mL of 95% ethanol, then add 436mg of 4-hydroxy-3-acetylcoumarin hydrazone, drop in 2 drops of glacial acetic acid, reflux under normal pressure for 3h, cool After reaching room temperature, a large amount of solid was precipitated, filtered under reduced pressure, and the filter residue was washed with 95% ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 72%.
Embodiment 3
[0034] Dissolve 195mg of 3,5-dimethylpyrrole-2-carbaldehyde in 10mL of 75% ethanol, then add 218mg of 4-hydroxy-3-acetylcoumarin hydrazone, drop in 2 drops of glacial acetic acid, reflux under normal pressure for 3h, cool After reaching room temperature, a large amount of solid was precipitated, filtered under reduced pressure, and the filter residue was washed with 75% ethanol to obtain a yellow solid, which was the target product, and the yield of the target product was 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com