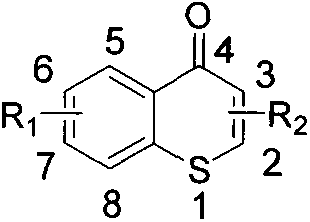
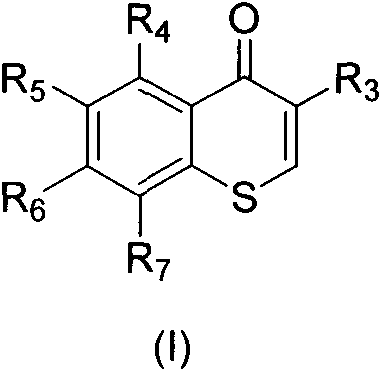
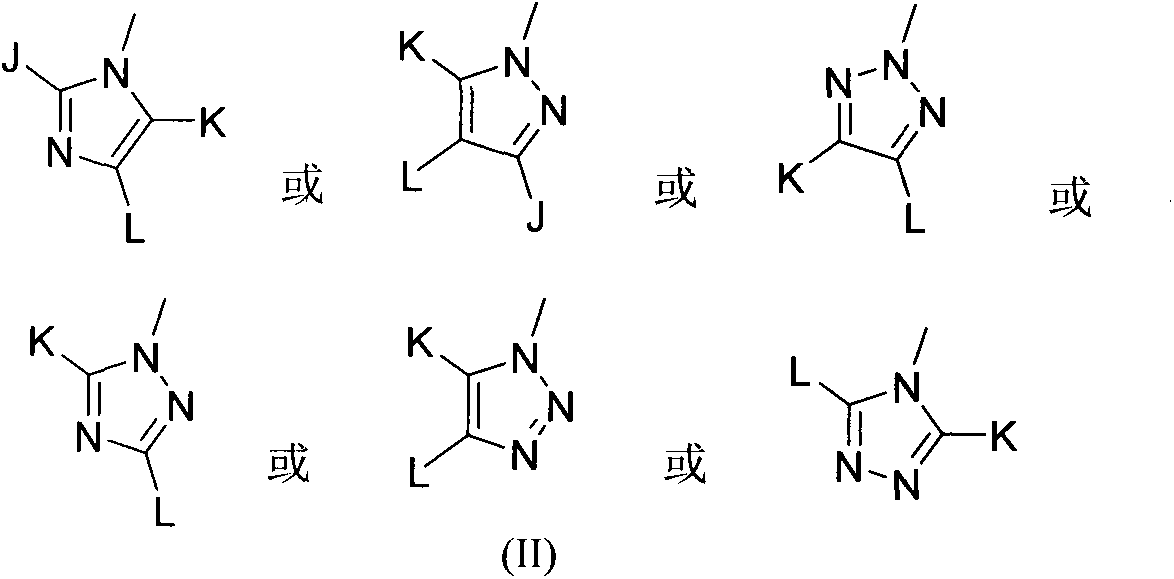
A kind of 3-azacyclic thiochromone compound and its synthesis method and application in antifungal drugs
A synthesis method and compound technology, applied in antifungal agents, botanical equipment and methods, medical preparations containing active ingredients, etc., can solve the problems of narrow antibacterial spectrum and increased difficulty in the treatment of fungal infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, the synthetic preparation reaction formula of formula (I) 3-oxazole thiochromone is as follows:
[0035]
[0036] The preparation steps are as follows:
[0037] Add 1-(2,4-difluorophenyl)-2-(1,2,4-triazol-1-yl)ethanone 18.4mmol, DMSO 30mL, NaOAc 43mmol, MnO 2 92mmol, stirred at 20°C, added CS dropwise at this temperature 2 22.8 mmol in 5 mL DMSO. After dropping, the temperature was controlled at 20°C and the reaction was stirred. After the reaction was completed, the reaction was filtered with suction, and the filtrate was extracted with ethyl acetate (50 mL×3) and water (50 mL). The organic phases were combined and evaporated under reduced pressure to obtain an off-white solid, which was recrystallized from anhydrous methanol to obtain the product of formula (I1) 3-(1,2,4-triazol-1-yl)-7fluorothiochromone--16.2 mmol, yield 88%.
[0038] 1 H NMR (300MHz, DMSO) δ9.16(s, 1H), 9.00(s, 1H), 8.62-8.44(m, 1H), 8.23(s, 1H), 8.03(d, J=8.9Hz, 1H) , 7.56...
Embodiment 2
[0039] Embodiment 2, the synthetic preparation reaction formula of formula (I) 3-oxazole thiochromone is as follows
[0040]
[0041] The preparation steps are as follows:
[0042] Add 1-(2,4-dichloro-5-fluorophenyl)-2-(imidazol-1-yl)ethanone 18.4mmol, DMSO 30mL, KOH 43mmol into a single-necked flask, stir at 40°C, drop Add CS 2 22.8 mmol in 5 mL DMSO. After the dropwise addition, the temperature was controlled at 40°C and the reaction was stirred for 3 h, and 100 mmol H 2 o 2 , continue to keep warm for 10h. After the reaction was completed, the reaction solution was extracted with ethyl acetate (50 mL×3) and water (50 mL). The organic phases were combined and rotary evaporated under reduced pressure to obtain an off-white solid, which was recrystallized from anhydrous methanol to obtain the product of formula (I2) 3-(imidazol-1-yl)-6-fluoro-7-chlorothiochromone--17.1mmol. The rate is 93%.
[0043] 1H NMR (300MHz, DMSO) δ8.77(s, 1H), 8.51(d, J=6.7Hz, 1H), 8.25(d, ...
Embodiment 3~ Embodiment 9
[0044] Embodiment 3~Example 9: Preparation of formula (I) 3-oxazole thiochromones
[0045] With (III) compound as raw material, prepare product formula (I) compound (target product is each compound of formula (I3)~(I9) in table 1), preparation step is the same as embodiment 1, and reaction formula is as follows
[0046]
[0047] In Examples 3 to 9, the selection of each group of the product formula (I) 3-azole thiochromones, the preparation reagents and the detection data are listed in Table 1.
[0048] Table 1
[0049]
[0050]
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com