Application of 7-hydroxycoumarin pyrazoline derivatives in the preparation of grp94 inhibitors
An inhibitor, pyrazole technology, applied in the field of coumarin pyrazoline fluorescent compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of 3-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-3-yl)-7-hydroxyl-2H-chromene-2-one
[0043] Dissolve 2,4-dihydroxybenzaldehyde (20mmol) and ethyl acetoacetate (24mmol) in 30ml of ethanol solution, and add 1ml of piperidine dropwise as a catalyst. The reaction solution was heated to reflux for 3h to the end of the reaction, and a large amount of grass-green solid was precipitated after cooling to room temperature. The crude product was obtained by suction filtration, and recrystallized with ethanol to obtain the green product 7-hydroxy-3-acetylcoumarin.
[0044] 7-Hydroxy-3-acetylcoumarin, benzaldehyde and a catalytic amount of piperidine (about 1ml) were dissolved in 20ml of absolute ethanol, and the reaction solution was refluxed for 4h and cooled to room temperature. Since the conversion rate of this reaction is low under weak alkaline conditions, 10 equivalents of benzaldehyde is added to react, and the reaction is carried out thoroughly. precipita...
Embodiment 2
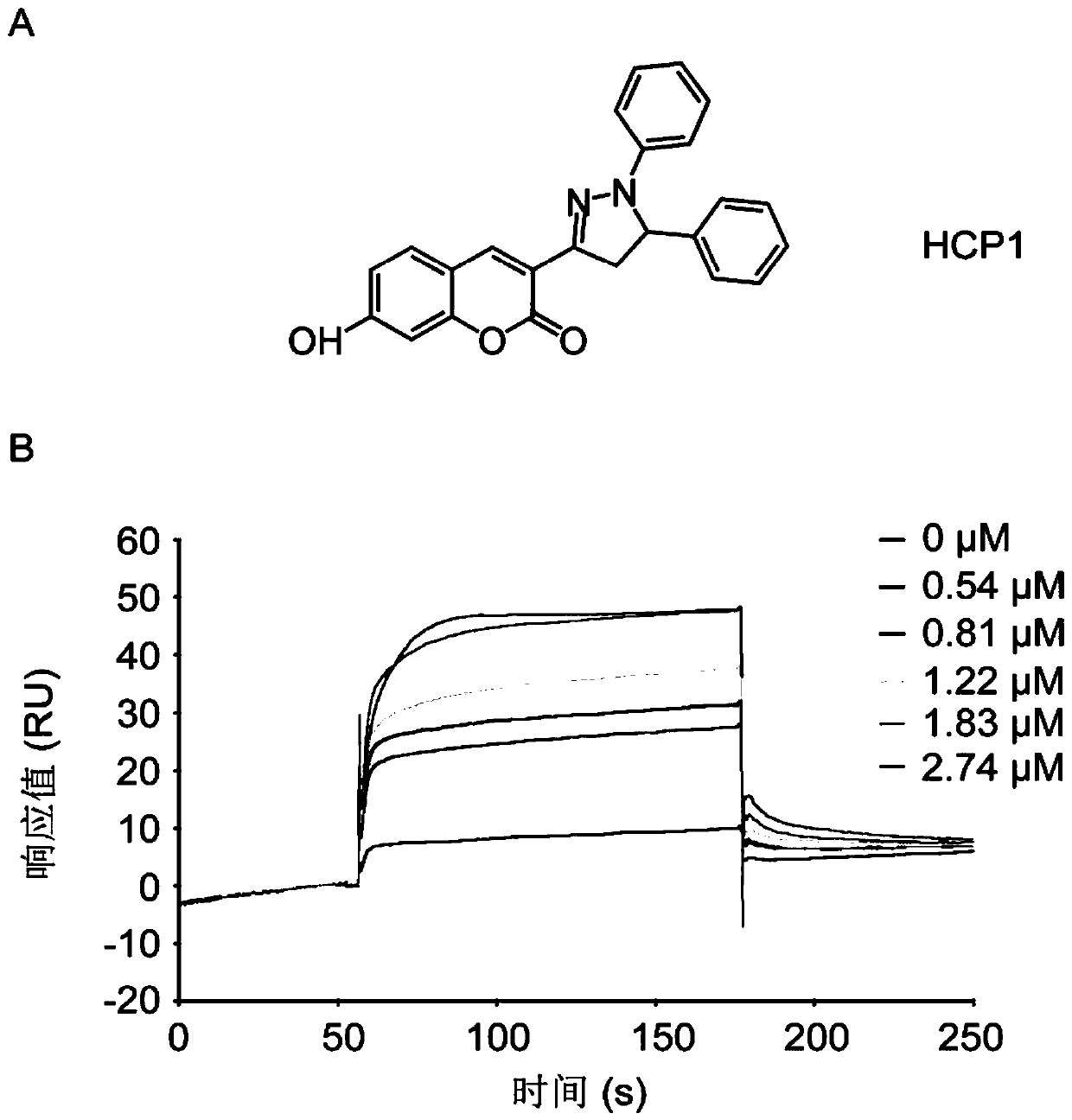
[0046] Example 2: HCP1 interacts directly with GRP94
[0047] Surface plasmon resonance analysis by Biacore T200 was used to detect the binding of HCP1 to full-length GRP94 protein. The full-length GRP94 protein was immobilized on the surface of the CM5 chip by amino coupling method. HCP1 was injected at 2.74 μM, 1.83 μM, 1.22 μM, 0.81 μM and 0.54 μM, respectively. The injection time of HCP1 is 120s, the flow rate is 10ul / min, and the dissociation time is 600s. The injection buffer was PBS (10 mM phosphate, 137 mM NaCl, 2.68 mM KCl, 0.1% dimethyl sulfoxide [v / v], pH 8.0), and the temperature was 25°C. Chip regeneration was performed using 10 mM NaOH after each injection. The combined response value was the RU value, and the blank control was deducted from the data processing, and the Biacore T200 evaluation software was used for 1:1 binding simulation analysis.
[0048] The results show that: the response value obtained by Biacore T200 has a concentration-corresponding rel...
Embodiment 3
[0049] Example 3: Colocalization of HCP1 and GRP94
[0050] A549 cells were seeded in glass culture dishes with a diameter of 3.5 cm at 37°C in CO 2 After culturing in the incubator for 24 hours, add HCP1 (10 μM) for 3 hours, remove the culture medium, wash twice with 0.1M PBS buffer, add 1640 stock solution, and place under a laser confocal microscope with different wavelengths of excitation light (405nm, 488nm, 546nm, 633nm and 647nm) to take pictures.
[0051] A549 cells were seeded in 3.5 cm diameter glass-bottom culture dishes at 37°C in CO 2 After culturing in the incubator for 24 hours, add HCP1 (10 μM) to treat for 3 hours, remove the culture medium, wash twice with 0.1M PBS, and fix the cells with 4% paraformaldehyde for 15 minutes. Discard 4% paraformaldehyde, wash 3 times with 0.1M PBS buffer, 5min each time; discard 0.1M PBS buffer, add normal serum blocking solution, block at room temperature for 20min; discard blocking solution, add GRP94 primary antibody (use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com