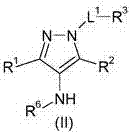
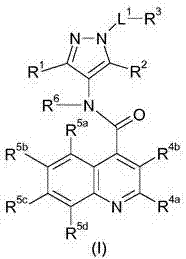
Glucose transport inhibitors
A technology of alkyl and group, which is applied in the field of intermediate compounds for the preparation of the compound, and can solve problems such as no specific disclosure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0988] N-[1-(4-fluorobenzyl)-3-methyl-1H-pyrazol-4-yl]-2,6-dimethylquinoline-4-carboxamide
[0989]
[0990] To 245 mg (1.19 mmol) 1-(4-fluorobenzyl)-3-methyl-1H-pyrazol-4-amine and 1-(4-fluorobenzyl)-5-methyl-1H-pyrazole -453 mg (1.19 mmol) HATU, 0.26 mL N , N - Diisopropylethylamine and 200 mg (0.99 mmol) of commercially available 2,6-dimethylquinoline-4-carboxylic acid. The reaction mixture was stirred at 25°C for 20 hours. The mixture was directly purified by preparative HPLC (Method A1) to obtain 208 mg (51%) of the desired title compound and 92 mg (23%) of the regioisomer N-[1-(4-fluorobenzyl)-5 -Methyl-1H-pyrazol-4-yl]-2,6-dimethylquinoline-4-carboxamide.
[0991] 1H NMR (500 MHz, DMSO d 6 ): δ (ppm) = 2.18 (s, 3H), 2.48 (s, 3H), 2.68(s, 3H), 5.25 (s, 2H), 7.19 (t, 2H), 7.36 (dd, 2H), 7.50 (s, 1H), 7.60 (dd, 1H), 7.80 (s, 1H), 7.89 (d, 1H), 8.23 (s, 1H), 10.21 (s, 1H).
Embodiment 2
[0993] 6,7-Difluoro-N-[1-(4-fluorobenzyl)-3-methyl-1H-pyrazol-4-yl]-2-(trifluoromethyl)quinoline-4-carboxamide
[0994]
[0995] Similar to Example 1), 222 mg (1.08 mmol) of 1-(4-fluorobenzyl)-3-methyl-1H-pyrazol-4-amine and 1-(4-fluorobenzyl)-5- A mixture of methyl-1H-pyrazol-4-amine (Intermediate 1C) and 250 mg (0.90 mmol) 6,7-difluoro-2-(trifluoromethyl)quinoline-4-carboxylic acid (Intermediate 5A ) reaction to give 137 mg (32%) of the desired title compound and 72 mg (17%) of the regioisomer 6,7-difluoro-N-[1-(4- fluorobenzyl)-5-methyl-1H-pyrazol-4-yl]-2-(trifluoromethyl)quinoline-4-carboxamide.
[0996] 1H NMR (400 MHz, DMSO d 6 ): δ (ppm) = 2.18 (s, 3H), 5.25 (s, 2H), 7.15- 7.22 (m, 2H), 7.35 (dd, 2H), 8.17 - 8.27 (m, 3H), 8.38 (dd, 1H), 10.44 (s,1H).
Embodiment 3
[0998] N-[1-(4-fluorobenzyl)-3-methyl-1H-pyrazol-4-yl]-2-methoxyquinoline-4-carboxamide
[0999]
[1000] Similar to Example 1), 303 mg (1.48 mmol) of 1-(4-fluorobenzyl)-3-methyl-1H-pyrazol-4-amine and 1-(4-fluorobenzyl)-5- A mixture of methyl-1H-pyrazol-4-amine (Intermediate 1C) was reacted with 250 mg (1.23 mmol) of commercially available 2-methoxyquinoline-4-carboxylic acid after purification by HPLC (Method C1) This gave 201 mg (37%) of the desired title compound and 97 mg (19%) of the regioisomer N-[1-(4-fluorobenzyl)-5-methyl-1H-pyrazol-4-yl ]-2-methoxyquinoline-4-carboxamide.
[1001] 1H NMR (500 MHz, DMSO d 6): δ (ppm) = 2.16 (s, 3H), 4.03 (s, 3H), 5.24(s, 2H), 7.16 - 7.21 (m, 3H), 7.31 - 7.37 (m, 2H), 7.47 (ddd, 1H), 7.71 (ddd, 1H), 7.84 (d, 1H), 7.97 (dd, 1H), 8.22 (s, 1H), 10.23 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com