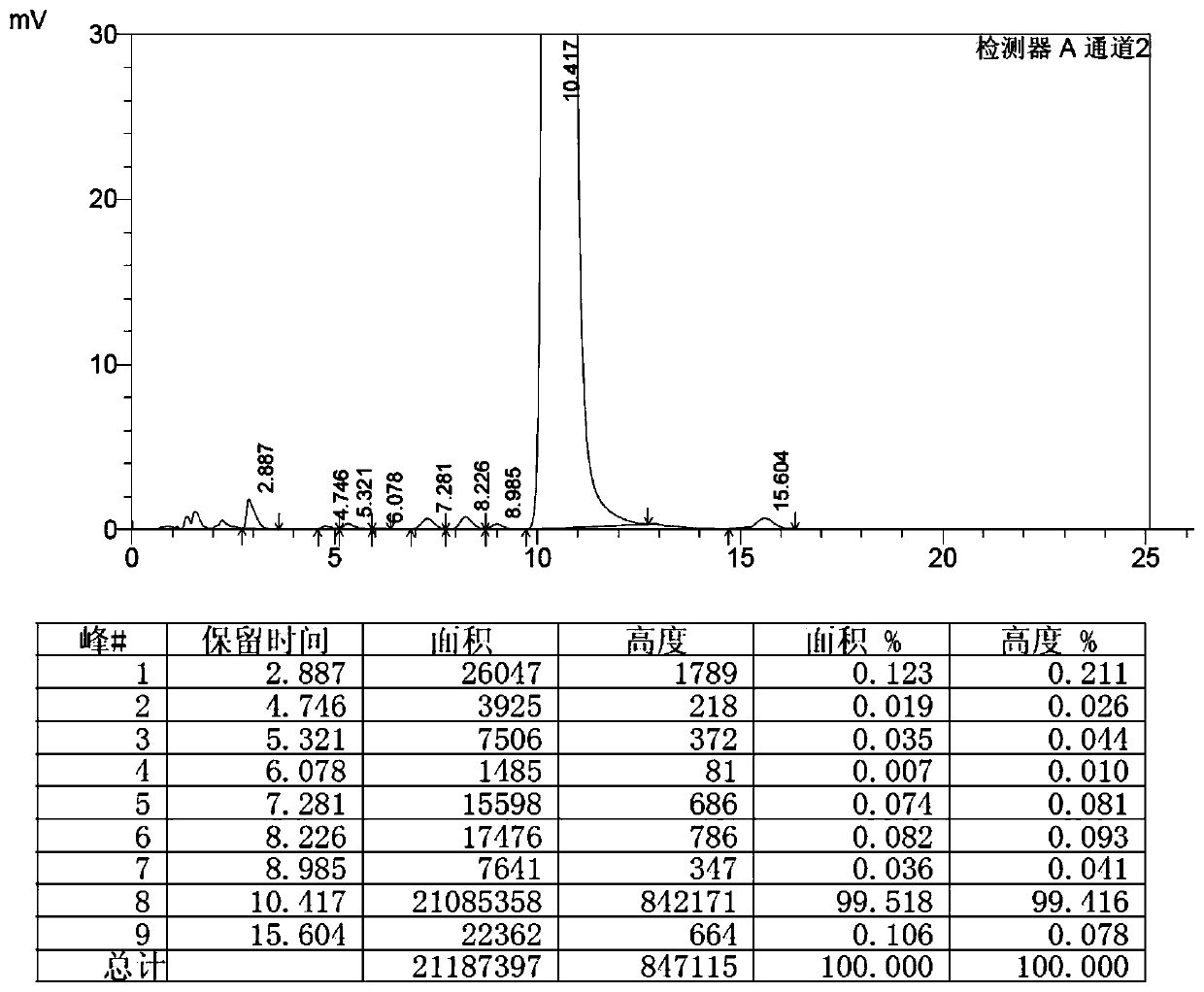
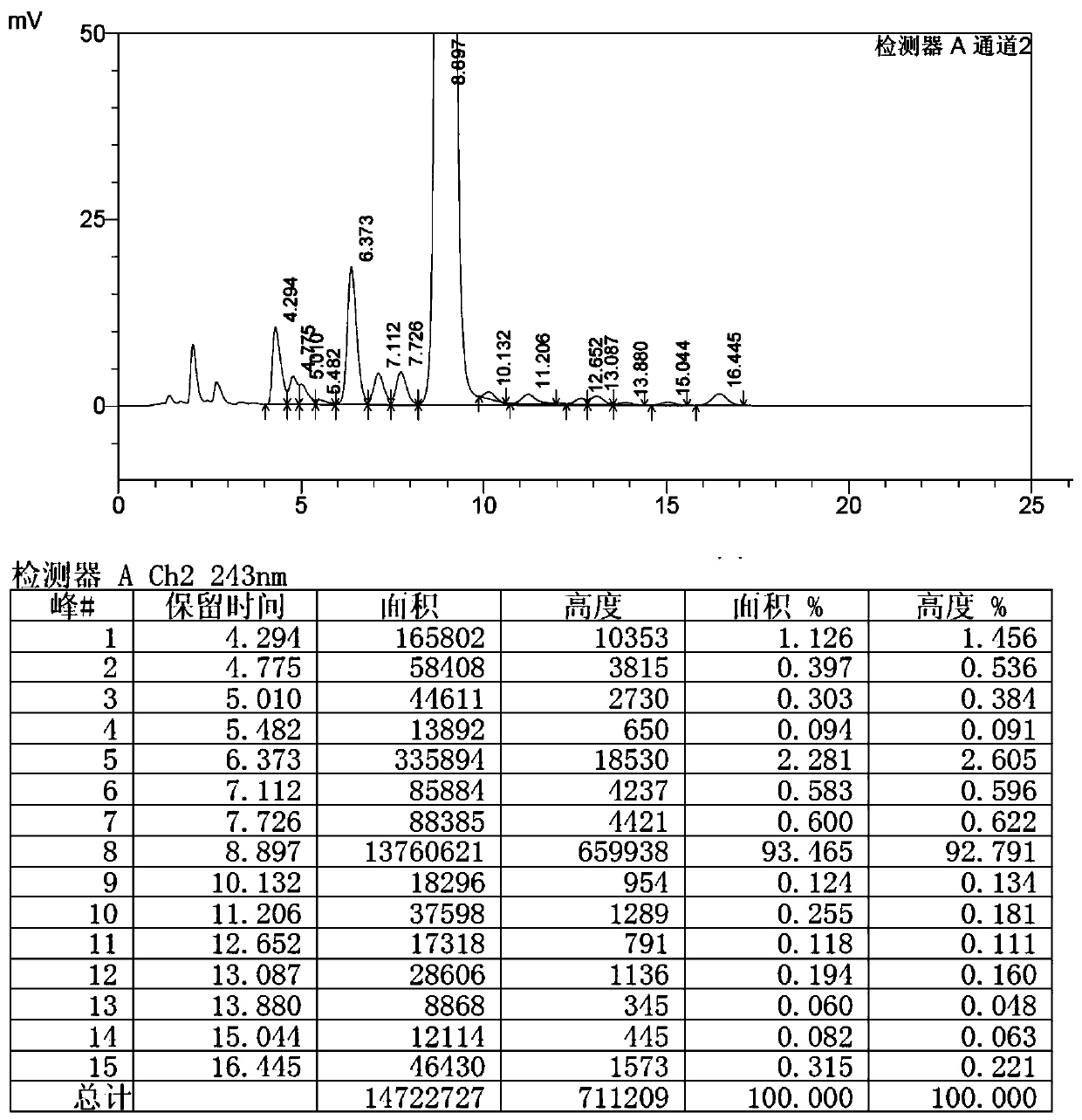
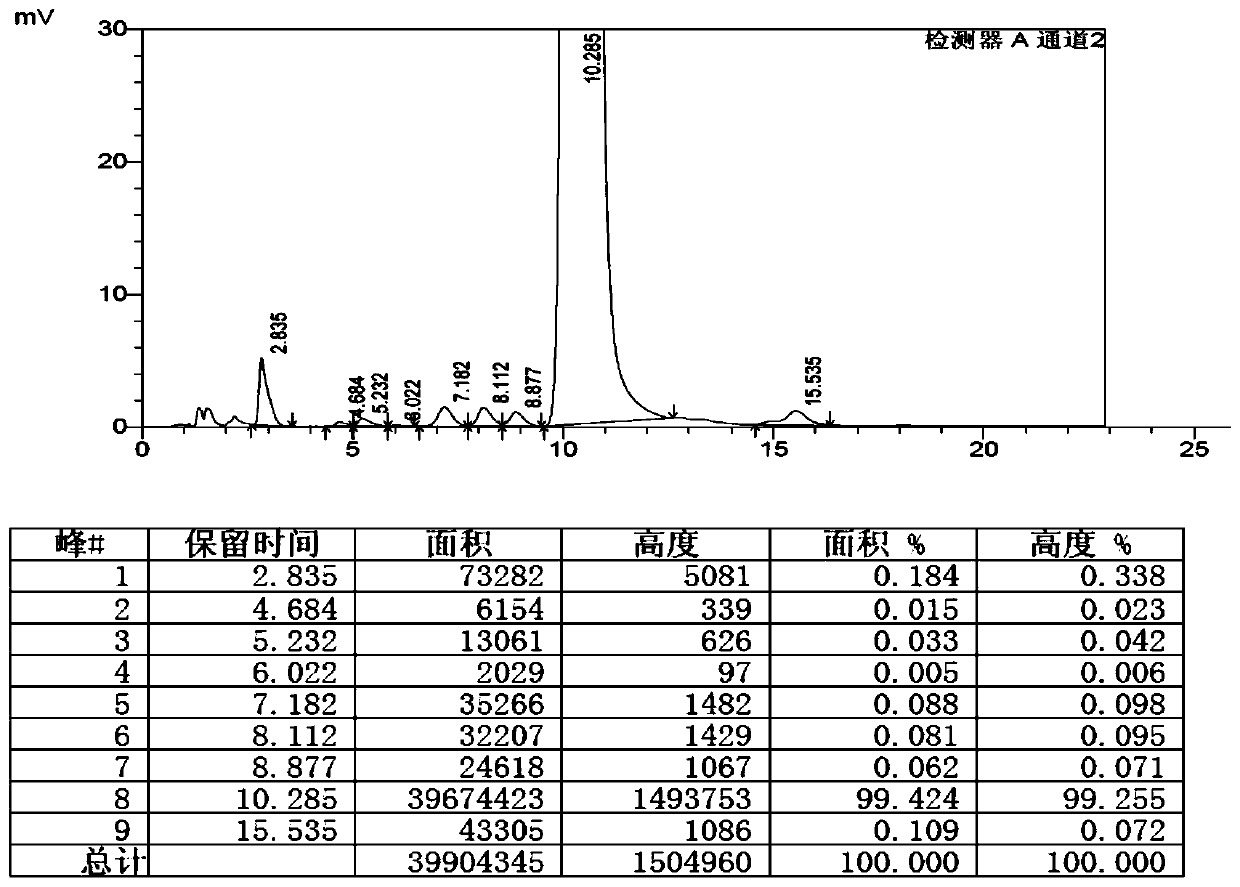
Preparation method of high-purity selamectin
A technology of selamectin and doramectin, which is applied in the field of preparation of high-purity selamectin, can solve the problem of low total yield of the process, low purity of selamectin crude product, and insufficient purity of selamectin Advanced problems, to achieve the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 SL1 crude product
[0048]
[0049] Add 40kg DL and 184kg acetone to the 300L hydrogenation kettle, vacuum N 2 Replace 3 times, stop stirring, add Wilkinson catalyst, N 2 Replaced 3 times, H 2 Replace 3 times. Start stirring, 35-40°C, hydrogen pressure 0.3-0.4Mpa, react for 3-4 hours, monitor by HPLC, DL raw material peak area 2 replacement. Concentrate under reduced pressure and evaporate to dryness in an external bath at 40-50°C with a water pump to obtain 40.5 kg of crude product with a purity of 88.8%.
Embodiment 2
[0050] The refining of embodiment 2 SL1
[0051] Add 81kg of methanol to 40.5kg of crude product, dissolve quickly, and stir for about 5 minutes to precipitate solids. Naturally cool down to room temperature, then cool down to 15-17°C and stir for 10-12 hours, centrifuge 39kg of wet product, and dry in a vacuum oven at 40°C in a water bath After 4 hours, 36 kg of SL1 crystalline solid was obtained with a purity of 96.6%.
Embodiment 3
[0052] Example 3 Preparation of SL2
[0053]
[0054] Add 36kg SL1 and 250kg isopropanol obtained in Example 2 to a 1000L stainless steel reactor, and stir in a turbid state at a temperature of 20.2°C. Slowly add 13.1kg of concentrated sulfuric acid dropwise for about 20 minutes. After the nitrogen breaks the vacuum, under nitrogen protection Keep warm at 22-24°C and stir for about 0.5h. The system is dissolved and stirred for 2 hours. HPLC monitoring, SL1<6%, stop the reaction, pour the reaction solution into 1000kg of ice water, add 500kg of dichloromethane for extraction, and then use 250kg of dichloromethane to extract the water layer Extract with methane, combine the organic layers, wash once with 5% sodium bicarbonate, and wash three times with 5% saline each time. Add 35 kg of anhydrous sodium sulfate to the organic phase and dry for 1 h, filter the solid with suction and rinse with 60 kg of dichloromethane, and concentrate the dry product to 36 kg with a purity of 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com