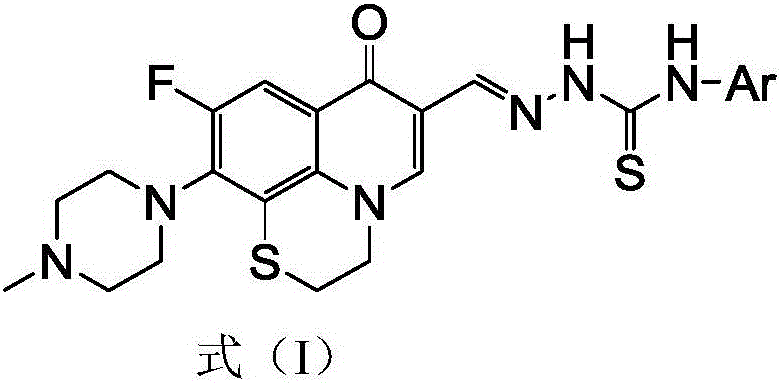
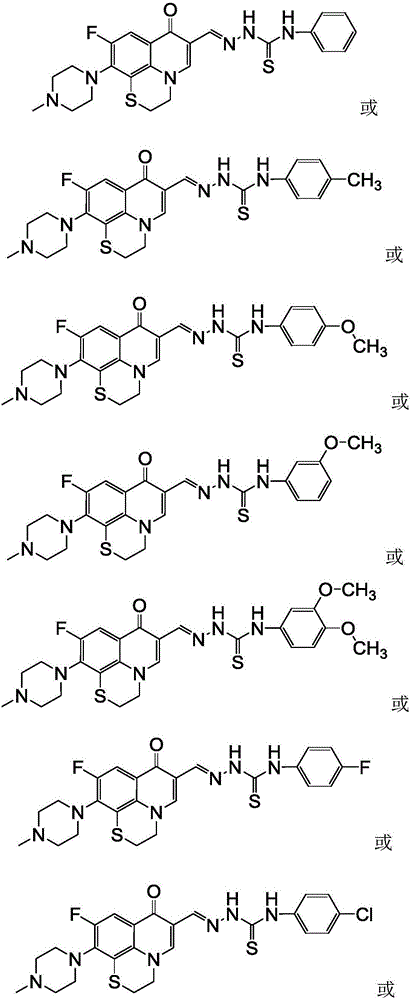
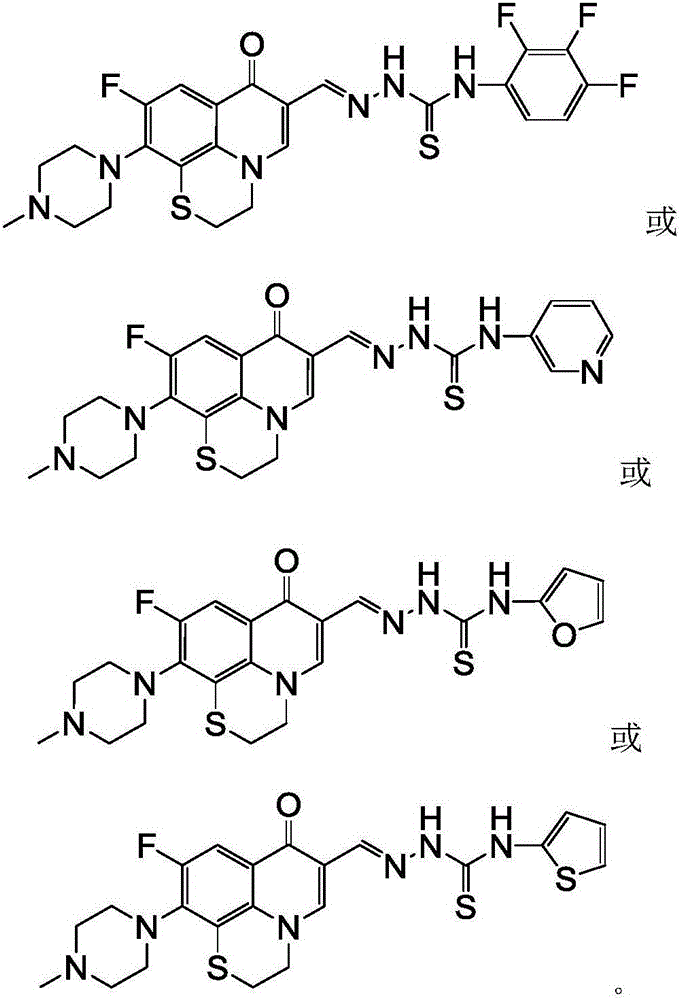
Rufloxacin aldolase 4-aryl thiosemicarbazides derivative and preparation method and application thereof
A technology of rufloxacin aldehyde and arylamino, applied in the field of innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] 3) The preparation method of 4-aryl thiosemicarbazides is as follows:
[0040] (a) carry out condensation reaction with the aromatic amines shown in formula (V) and carbon disulfide in the mixed solvent of ethanol-ammonia water to make the arylaminodithioformic acid ammonium (VI) shown in formula (VI);
[0041] (b) condensation reaction of arylaminodithioformate ammonium (VI) shown in formula (VI) and sodium chloroacetate to obtain S-carboxymethyl arylaminodithiocarbamate sodium salt shown in formula (VII);
[0042] (c) S-carboxymethyl arylaminodithiocarbamate sodium salt shown in formula (VII) and hydrazine hydrate undergo a substitution reaction, and the 4-aryl thiosemicarbazide shown in formula (VIII) is obtained through aftertreatment kind;
[0043]
[0044] For the specific operation steps, please refer to the preparation method of the literature "Synthesis and antibacterial activity of nitrogen-containing heterocyclic aryl thiosemicarbazones" (Hou Linyan, mast...
Embodiment 1
[0050] 6-Fluoro-8,1-(thioethyl)-7-(4-methyl-piperazin-1-yl)-quinolin-4(1H)-one-3-aldehyde acetal 4-phenylaminothio Urea (I-1), its chemical structural formula (I-1) is:
[0051]
[0052] That is, Ar in formula I is phenyl.
[0053] The preparation method of this compound is: the rufloxacin C-3 aldehyde crude product (1.0g) shown in formula (IV) is dissolved in dehydrated alcohol (20 milliliters), adds 4-phenylthiosemicarbazide (0.6g, 3.6 mmol) (that is, Ar in the 4-arylthiosemicarbazides shown in formula (VIII) is a phenyl group), refluxed for 12 hours, filtered while hot and collected the solid, and the gained solid was washed 2 times with ethanol and washed with distilled water successively Twice, dry, and recrystallize with a mixed solvent of DMF-ethanol with a volume ratio of 5:3 to obtain light yellow crystals with a structural formula such as formula (I-1), a mass of 0.72 g, and m.p.246 to 248°C;
[0054] 1 H NMR (400MHz, DMSO-d 6 )δ: 11.77 (s, 1H, CH=N), 9.96 (s, 1...
Embodiment 2
[0057] 6-Fluoro-8,1-(thioethyl)-7-(4-methyl-piperazin-1-yl)-quinolin-4(1H)-one-3-aldehyde acetal 4-(4-methyl Base phenyl) thiosemicarbazide (I-2), its chemical structural formula (I-2) is:
[0058]
[0059] That is, Ar in formula I is 4-methylphenyl.
[0060] The preparation method of this compound is: the rufloxacin C-3 aldehyde crude product (1.0g) shown in formula (IV) is dissolved in dehydrated alcohol (20 milliliters), adds 4-(4-methylphenyl)aminosulfur Urea (0.6g, 3.3mmol) (that is, Ar in the 4-arylthiosemicarbazides shown in formula (VIII) is 4-methylphenyl), refluxed for 12 hours, filtered while hot and collected the solid, the obtained The solid was washed twice with ethanol and distilled water twice, dried, and recrystallized with a mixed solvent of DMF-ethanol with a volume ratio of 5:3 to obtain a light yellow crystal with a structural formula such as formula (I-2) and a mass of 0.63 g, m.p.223~225℃;
[0061] 1 H NMR (400MHz, DMSO-d 6 )δ: 11.76(s, 1H, CH=N)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com