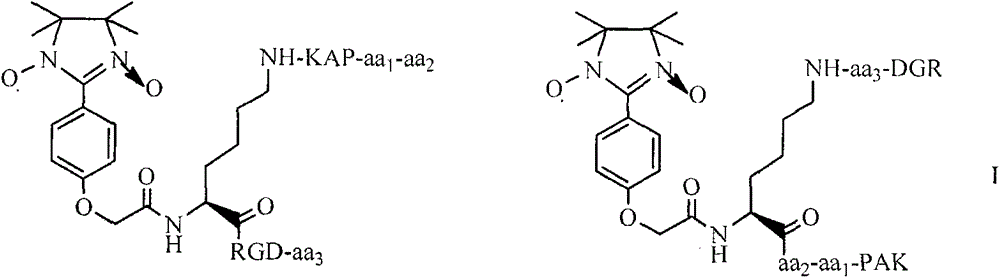
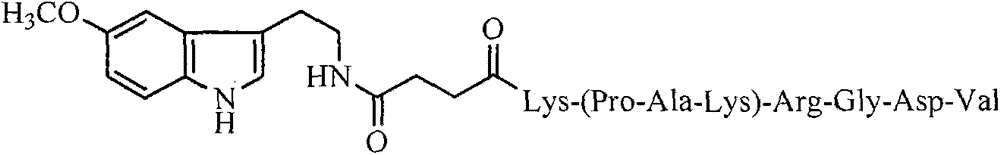Pentamethoxytryptophanylcarbonylpropionyl-PAK peptide, and preparation method, activities and applications thereof
A technology of pentamethoxytryptamine and carboxypropionyl, which is applied in the field of biomedicine and can solve problems such as no effective drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
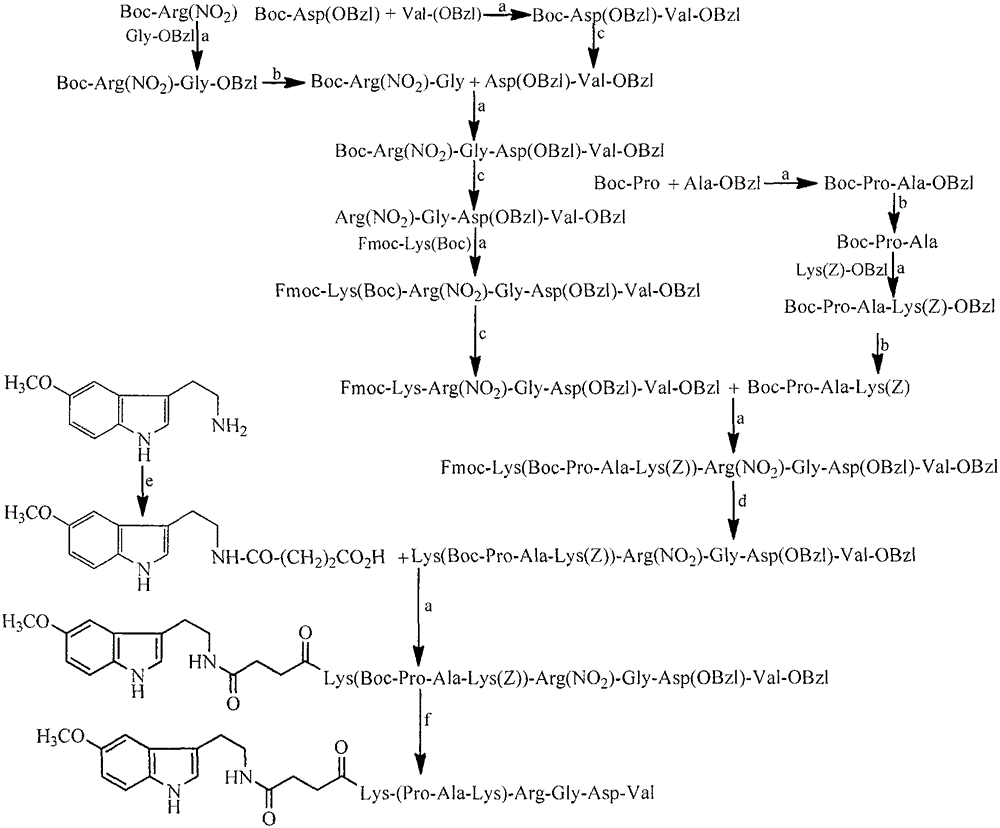
[0028] Embodiment 1 connects peptide general method
[0029] Dissolve 1mmol carboxy-terminal compound in dry THF, add 1.2mmol N-hydroxybenzotriazole (HOBt) and 1.2mmol N,N-dicyclohexylcarbodiimide (DCC) dissolved in dry THF successively under stirring in an ice bath , stirred for 0.5h, dissolved 1.05mmol of the amino-terminal compound in dry THF, added to the above reaction solution, adjusted the pH to 9 with N-methylmorpholine (NMM), stirred at room temperature for 6h, TLC (CHCl 3 / CH 3 OH, 10 / 1) showed that the carboxy-terminal raw material disappeared completely, and the reaction ended. DCU was removed by filtration, the filtrate was concentrated under reduced pressure and dissolved in ethyl acetate, and the resulting solution was successively washed with saturated NaHCO 3 Wash 3 times with aqueous solution, 3 times with saturated NaCl aqueous solution, 5% KHSO 4 Wash 3 times with aqueous solution, 3 times with saturated NaCl aqueous solution, 5% NaHCO 3 Wash 3 times wi...
Embodiment 2
[0030] Embodiment 2 removes N-tert-butoxycarbonyl protecting group general method
[0031] Dissolve 1 mmol of the compound containing N-tert-butoxycarbonyl protecting group in a small amount of dry ethyl acetate, add 10 mL of 4N hydrogen chloride / ethyl acetate solution under ice-bath stirring, stir in ice-bath for 1-2 h, TLC (CHCl 3 / CH 3 OH, 10 / 1) showed complete disappearance of the starting material and the reaction was complete. The reaction solution was concentrated under reduced pressure. The residue was added with 5 ml of anhydrous ethyl acetate, and the solution was concentrated to dryness under reduced pressure. This operation was repeated 3 times. The residue was added with 5 ml of anhydrous diethyl ether, and the solution was concentrated to dryness under reduced pressure. This operation was repeated 3 times. The obtained target compound was directly used in the next step reaction.
Embodiment 3
[0032] Embodiment 3 hydrolysis removes benzyl ester protecting group general method
[0033] Dissolve the compound containing the benzyl ester protecting group in methanol, slowly add 2M NaOH aqueous solution dropwise under ice bath and stirring, adjust the pH to 12, react for 5h, TLC (CHCl 3 / CH 3 OH, 10 / 1) showed complete disappearance of the starting material and the reaction was complete. Slowly add saturated KHSO dropwise under stirring in an ice bath 4 The aqueous solution was adjusted to pH 7, concentrated under reduced pressure to remove methanol, and the remaining aqueous solution was slowly added dropwise with saturated KHSO under stirring in an ice bath. 4 The aqueous solution was adjusted to pH 3, extracted 3 times with ethyl acetate, the combined ethyl acetate layer was washed 3 times with saturated NaCl aqueous solution, washed with anhydrous NaCl 2 SO 4 Dry, filter, and concentrate the filtrate under reduced pressure to obtain the target compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com