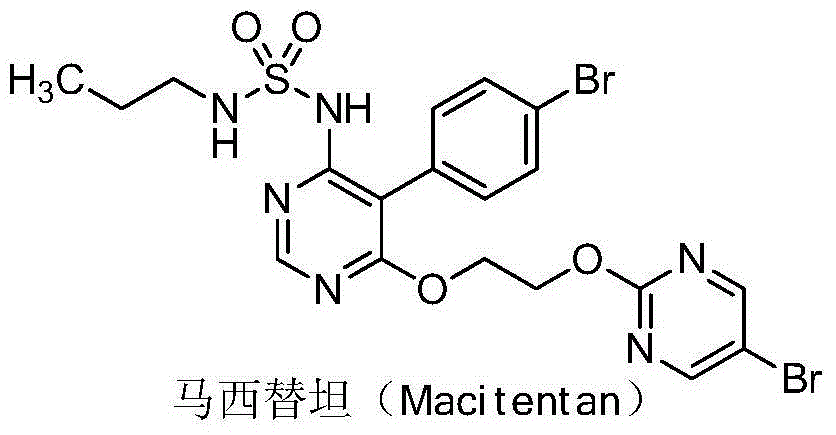
Method for preparing macitentan related substance
The technology of a compound, bromophenyl, is applied in the field of preparation of macitentan-related substances, which can solve the problems such as the synthesis method of the compound of formula (I) that has not been reported, and achieve the effects of rapid preparation, simple purification method and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add macitentan (1.2g), methanol (40mL) into a 250mL three-neck flask, heat to 60°C for 24 hours, and detect by HPLC, the reaction solution contains 5.1% of the compound of formula (I), 87.2% of macitentan .
Embodiment 2
[0029] Add macitentan (1.2g) and ethylene glycol (40mL) into a 250mL three-neck flask, heat to 80°C for 24 hours, and detect by HPLC, the reaction solution contains 10.2% of the compound of formula (I), 79.9% of macitentan Titan.
Embodiment 3
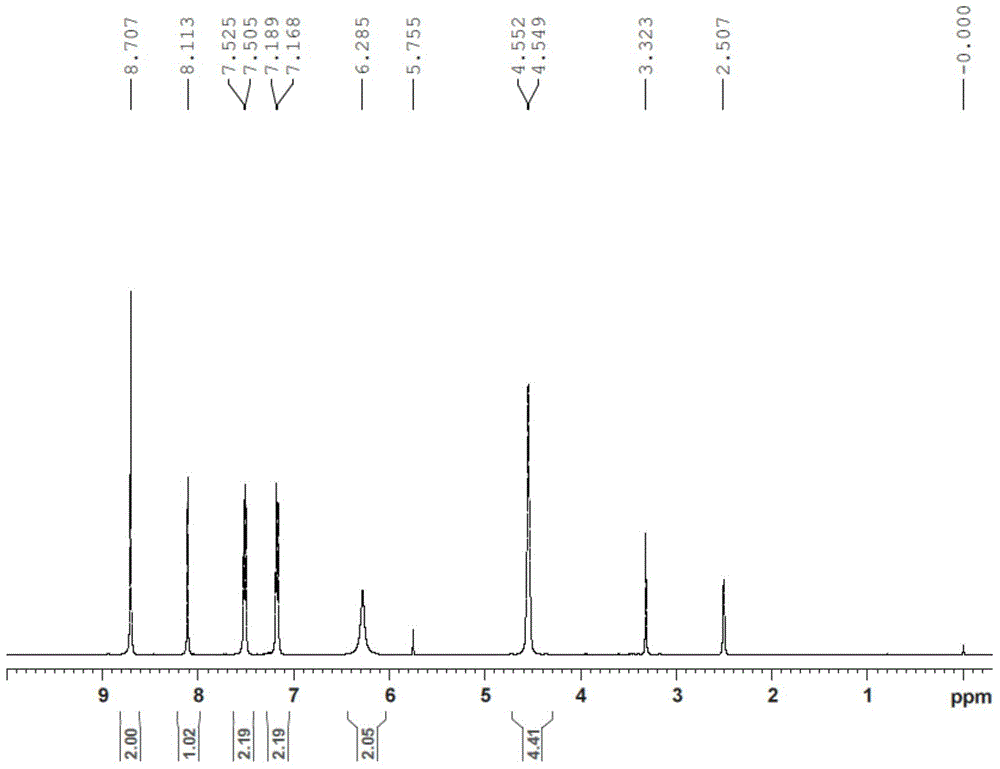
[0031] Add macitentan (1.2 g) and ethylene glycol (40 mL) into a 250 mL three-necked flask, and heat to 110° C. for 3 hours. Add water (200mL) to the reaction solution, extract with dichloromethane (100mLx2), wash the organic layer with water (50mL) and brine (50mL), combine the organic phases, dry over anhydrous sodium sulfate, filter and concentrate under reduced pressure to obtain the formula (I) Crude compound. Add methanol (50 mL) to the crude compound of formula (I) and stir for 2 hours, filter with suction, and vacuum-dry the filter cake to obtain compound of formula (I) (0.5 g). The purity of the product is 99.5% as detected by HPLC. (a, MS-ESI(m / z): 465.9[M+H] + ; b. 1 H-NMR (DMSO-d 6 )δ:8.70(s,2H),8.11(s,1H),7.51-7.53(d,J=8Hz,2H),7.17-7.19(d,J=8.0Hz,2H),6.29(s,2H) ,4.55(br,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com