Fgf-18 in graft transplantation and tissue engineering procedures
A technology of FGF-18 and tissue engineering, applied in the field of regenerative medicine, can solve the problems such as the speed and quality of cartilage incorporation need to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] method:
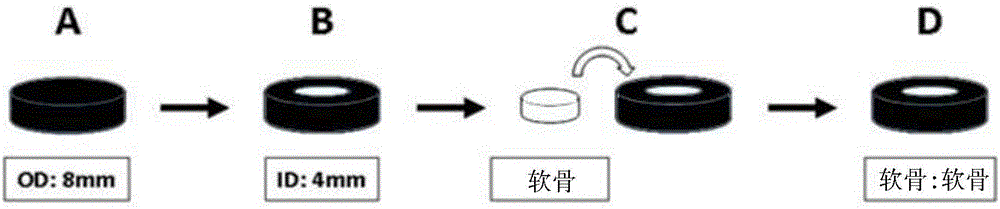
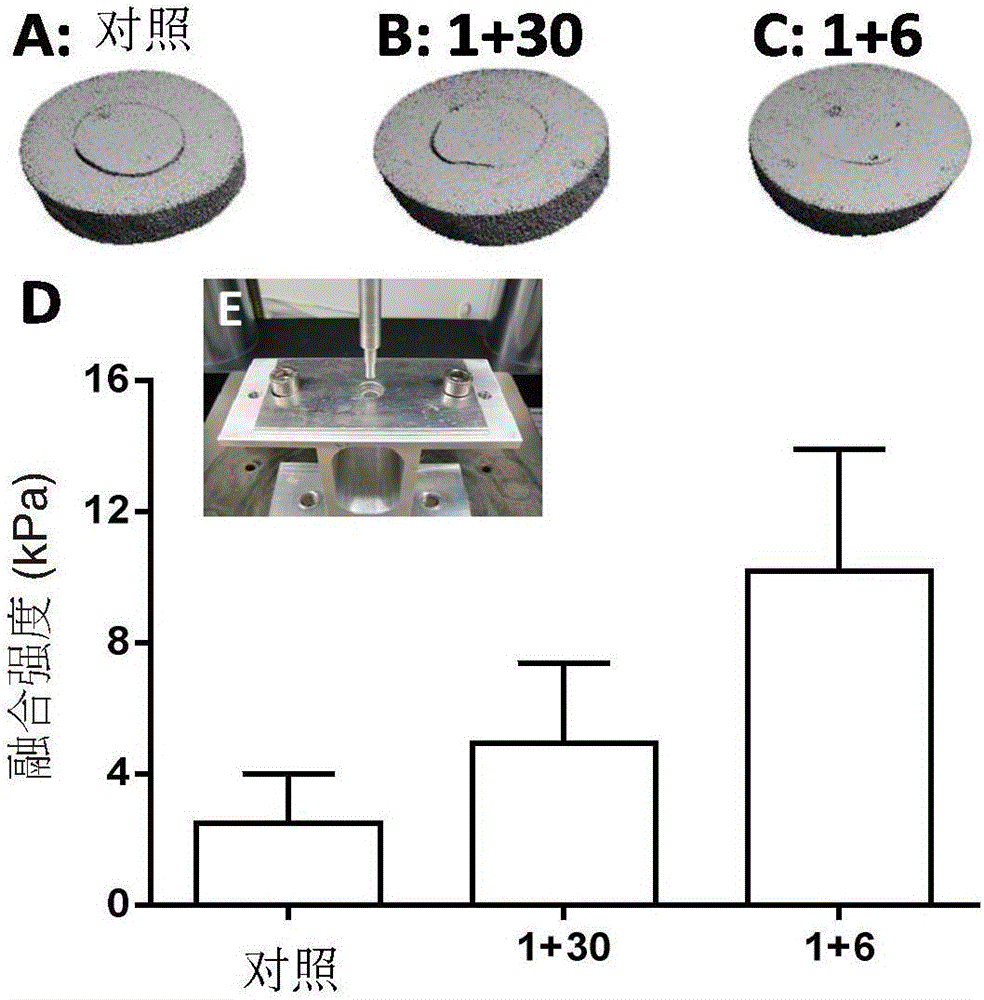
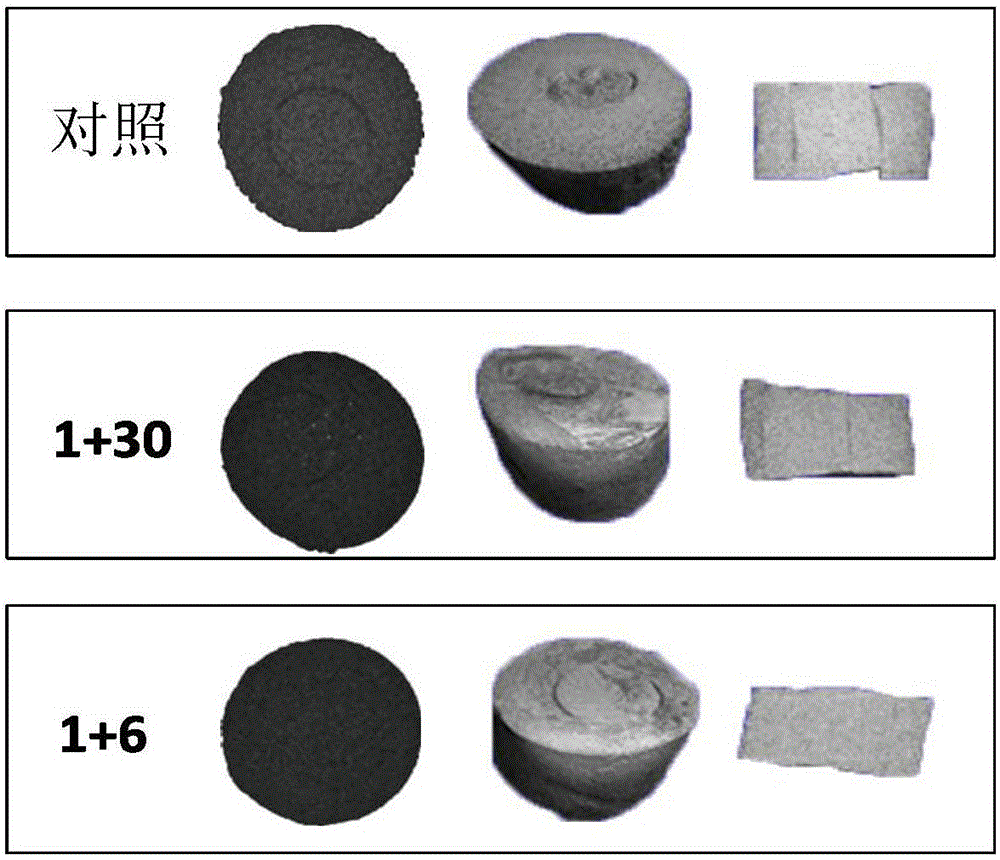
[0077] Fresh hyaline cartilage was collected from the trochlear groove of juvenile achyranthes knees (3-6 months old). An 8 mm cylindrical explant was removed through a biopsy punch ( figure 1 A), and in complete medium (DMEM 4.5g / L D-glucose and L-glutamine, 10% FBS, 1% PSF, 1% amphotericin B (Fungizone), 1% MEM vitamins, 25mM HEPES and 50 μg / ml vitamin C) overnight. Sample bone removal and defect construction (4mm diameter) to form a core ring repair structure ( figure 1 B). The inner core and outer ring were cultured separately for 24 hours, and then the original core was used to fill the defect. Samples were then cultured in complete medium or treated with Sprifermin (rhFGF18, 100 ng / ml). Treatment consisted of administration of rhFGF18 for 24 hours, once a week (and repeated weekly) (1+6), or 24 hours of treatment followed by culture in complete medium for 1 month (1+30 days). Samples were collected after 4 weeks of culture. Using a custom test b...
Embodiment 2
[0084] method:
[0085] Isolation of native osteoarthritic chondrocytes from patients undergoing total joint replacement. Cells were grown in monolayer culture for several days and then in scaffold-free 3D culture for a week before initiating treatment. The treatment consisted of continuous incubation with rhFGF-18 [100 ng / mL] or one day per week for four weeks. The results were compared to a control medium without sprifermin. The three-dimensional structure was characterized using biochemical assays, quantitative PCR (qPCR) and histology.
[0086] Results (data not shown):
[0087] To ensure maintenance of the phenotype, 3D scaffold-free medium was used to test the effect of sprifermin on hOA chondrocytes. In this setting, rhFGF-18 was demonstrated to have a beneficial effect [1 day per week] on cell content and significantly increased the size and matrix content (GAG and HPro content) of 3D structures. It was also found that rhFGF18 reduced collagen I expression compare...
Embodiment 3
[0091] method:
[0092] Porcine chondrocytes were isolated from femoral head cartilage of porcine hip. After joint dissection, cartilage was collected and digested with collagenase 0.25% for 45 min. Loose cells were discarded, and the cartilage was further digested with collagenase 0.1% overnight to extract chondrocytes. Porcine chondrocytes were cultured in suspension as CTA (cartilage tissue analogue) without any treatment for the first week, followed by one of the following treatments: 1) four weeks in medium continuously containing 10 or 100 ng / mL rhFGF18, 2) ) culture medium containing 10 or 100 ng / mL rhFGF18 for one week, followed by culture medium without rhFGF18 for three weeks, 3) medium supplemented with 10 or 100 ng / mL rhFGF18 on 1 day per week (i.e. contact 24 hours, followed by culturing in a medium without rhFGF18 for 6 days) for three weeks, or 4) in a medium without rhFGF18 for four weeks, as a control group ( Figure 4 ). At the end of the culture period, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com