Method for simultaneous determination of levo-glucan, mannan and galactan in aerosol by high performance liquid chromatography-tandem quadrupole mass spectrometry
A technology of high performance liquid chromatography and levodextran, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., to achieve the effect of reducing use, saving scientific research costs, and good detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
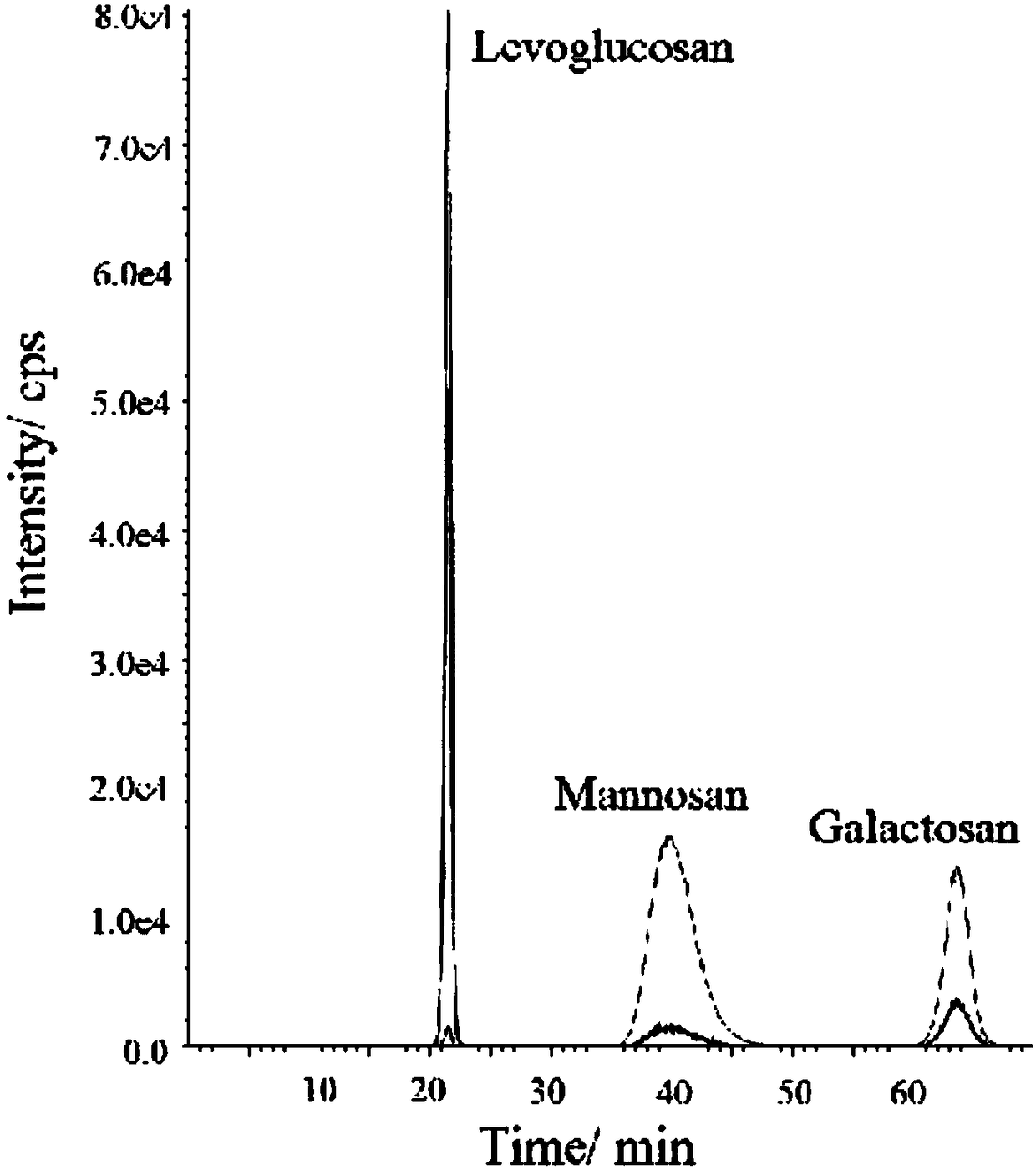
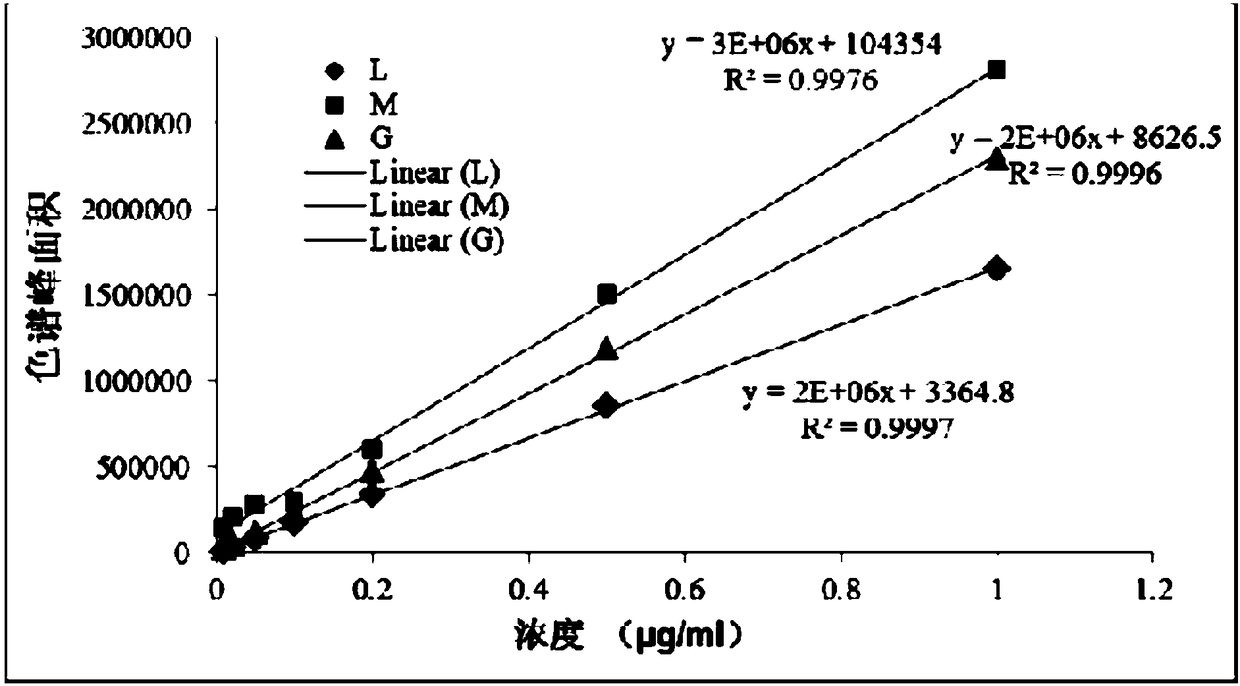
[0035] Precisely weigh 1.0 mg each of levoglucosan, galactan, and mannan, dissolve with an appropriate amount of ultrapure water, transfer to a 100ml volumetric flask, and dilute to the mark with ultrapure water. Shake well and let stand to obtain 10μg / ml L, M, G mixed standard solution. The solutions used for the test working curves of L, M, and G are diluted step by step from the above 10μg / ml standard solution, respectively prepared into a series of solutions of 0.005, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 1μg / ml, and then press the above Experimental conditions: High performance liquid chromatography-tandem quadrupole mass spectrometer HPLC-MSMS analysis and detection, the mobile phase is selected as ammonia water and ultrapure water (10:90, v / v) with a mass fraction of 0.001%, and the constant flow rate is set to 0.4 ml min -1 , the temperature of the column oven was 45°C. The optimal ion spray voltage is set to -3800V, the atomizing gas is set to 38psi, the atomizing temper...
Embodiment 2
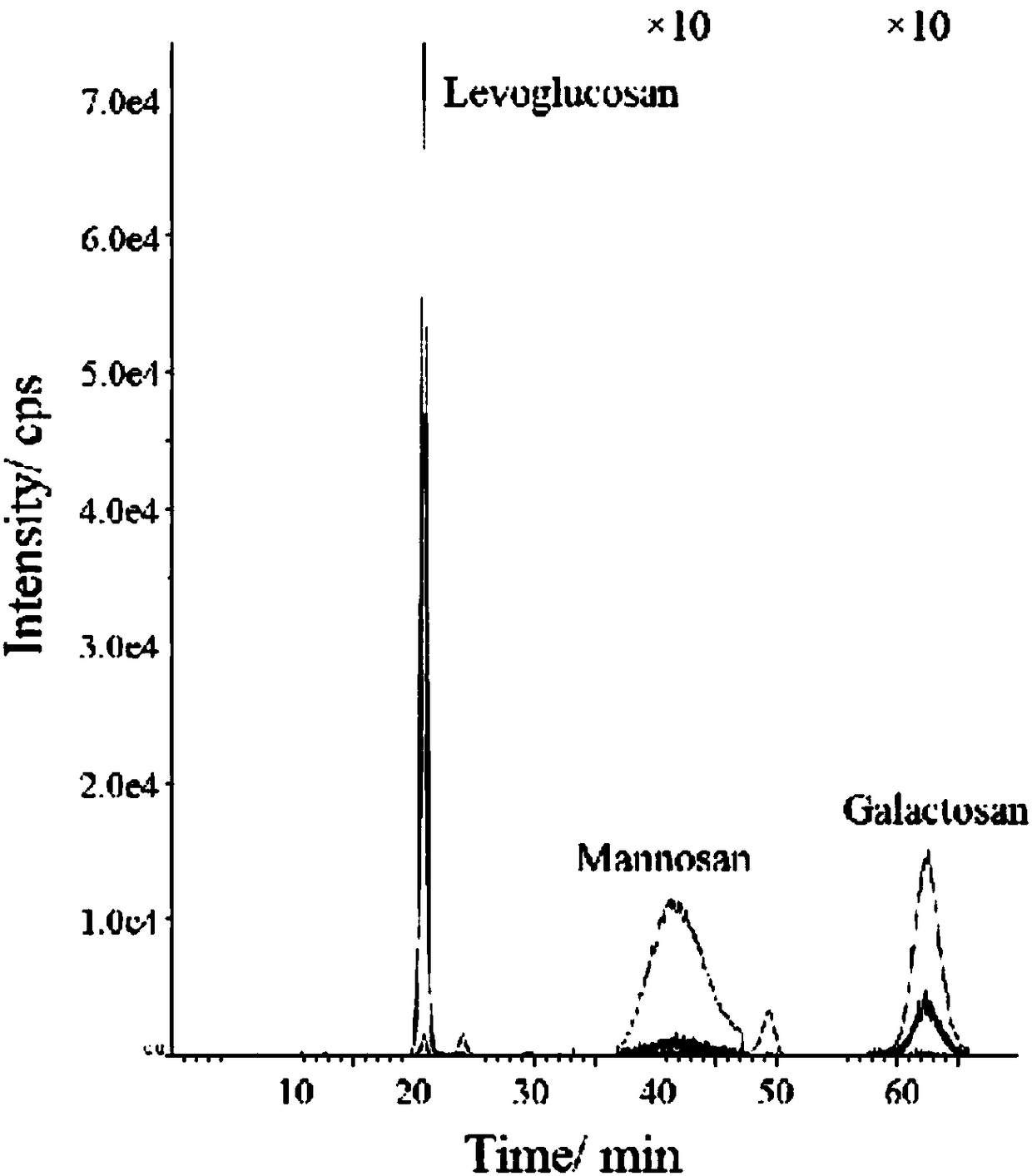
[0037] Cut the high-flow air particle sampling filter membrane 3cm×3cm, cut it into pieces, add 3ml ultrapure water, and perform ultrasonic extraction for 45 minutes. After the extract was filtered through a 0.45 μm hydrophobic PTFE needle filter, the filtered extract was tested and analyzed with a high performance liquid phase-tandem quadrupole mass spectrometer. The test analysis experiment condition is the same as embodiment 1, external standard method is quantitative. Three parallel samples were made to the air aerosol sample, the test numbers are A, B, and C, the analysis results are shown in Table 2, and the first parallel sample (test number A) was measured 5 times, and the analysis results are shown in Table 3. As can be seen from Table 2, the relative standard deviation (RSD) of L in the parallel sample is 8.98%, the relative standard deviation of M is 12.43%, and the relative standard deviation of G is 6.98%; The relative standard deviation is 1.31%, the relative st...
Embodiment 3
[0044] The standard solution in Example 1 was added to the prepared test solution with known negative results (the test did not contain the target compound), and the experiment was carried out according to the instrumental analysis and detection method in Example 1. This sample is done 10 parallel measurements to get the average value, according to the actual addition and the measured results, calculate the recovery rate of the sample, the test results are shown in Table 4, as can be seen from Table 4, the recovery of the standard addition of levoglucosan in the sample The rate reaches between 97-101%, and the relative standard deviation of parallel measurement is 0.95%; The spiked recovery of lactan was between 99-100%, and the relative standard deviation of parallel measurement was 0.47%;
[0045] Table 4 The recovery of standard addition of this sample
[0046]
[0047]
[0048] figure 1 It is the chromatogram of L, M and G detected in Example 1 of the present inven...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com