Nucleotide sequence and application thereof
A nucleotide sequence, Epstein-Barr virus technology, applied in nucleic acid vectors, medical preparations containing active ingredients, introduction of foreign genetic material using vectors, etc., can solve the problem of no Epstein-Barr virus subunit vaccine report and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
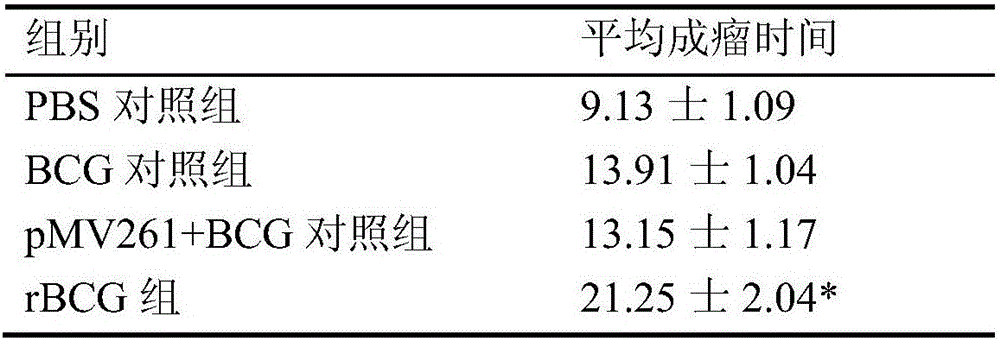
Image
Examples
Embodiment 1
[0020] The synthetic process of embodiment 1 nucleotide sequence SEQ ID No.1
[0021] The nucleotide sequence of SEQ ID No.1 is composed of the cDNA sequence of IL-2, the cDNA sequence of LMP2A and the fusion fragment. The nucleotide sequence of the cDNA sequence of IL-2 is shown in SEQ ID No.2. The cDNA of LMP2A The nucleotide sequence of the sequence is shown in SEQ ID No.3, and the nucleotide sequence of the fusion fragment is shown in SEQ ID No.4. The specific synthesis process of the nucleotide sequence of the present invention is as follows:
[0022] Using IL-2 cDNA as a template, upstream primer F1, downstream overlapping primer F2, PCR amplification, pre-denaturation at 95°C for 5 minutes; denaturation at 95°C for 30 seconds, annealing at 60°C for 45 seconds, extension at 72°C for 2 minutes; denaturation-annealing-extension process 60 cycles were performed, with a final extension of 10 min at 72°C.
[0023] The IL-2 fragment is obtained, the nucleotide sequence of F1...
Embodiment 2
[0026] Embodiment 2 Connection and transformation of fusion gene LMPI-2 and plasmid pMV261
[0027] After the purified gene LMPI-2 and plasmid pMV261 were digested with EcoR I and Sal I, they were ligated; 0.1-0.3 pmoL of LMPI-2 gene fragment, 1 μL of pMV261, T 4 DNA ligase 1.5 μL with ddH 2 O was adjusted to 5 μL, then 5 μL of ligation buffer was added, and reacted overnight at 16°C.
[0028] Apply 50 μl of isopropylthiogalactopyranoside (IPTG) on the prepared bacterial basal medium LB solid plate, and place it at room temperature for 2 hours. Take out the competent E.coli DH5α cells from the ultra-low temperature refrigerator and thaw in the ice bath. Take 30-40 μl of E.coliDH5α cells and gently mix the recombinant plasmid, ice-bath for 20-30 minutes, heat shock at 42°C for 90 seconds, immediately ice-bath for 3 minutes; add 1ml of liquid LB medium (without antibiotics), and incubate at 37°C for 2 hours; 12 Centrifuge at 000r / min (radius 10cm) for 3min, and redissolve the...
Embodiment 3
[0029] Construction and expression of embodiment 3 recombinant BCG
[0030] Preparation of Bacillus Calmette-Guérin (BCG) Competent: BCG (Bacillus Calmette-Guérin, referred to as BCG, Chinese name comes from its inventor Karl-Jie) is a vaccine for the prevention of tuberculosis, using live avirulent Mycobacterium bovis (Mycobacterium bovis) made.
[0031]BCG was cultured in Middlebrook7H9 medium at 37°C on a shaker (rotating speed: 150rpm); when the OD600 value of the culture solution was about 0.6, add the BCG bacterial liquid into the centrifuge tube and put it in an ice bath for 2 hours; Pre-cool for 2 hours; centrifuge at 4°C for 15 minutes at 8,000 rpm, discard the supernatant; resuspend the bacteria with 1 / 10 of the original volume and pre-cooled 10% glycerol; repeat the above operation for a total of five times; discard the supernatant, 1 / 50 of the original volume was added to resuspend the bacterial cells in 10% glycerol to obtain competent bacteria, which were placed...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap