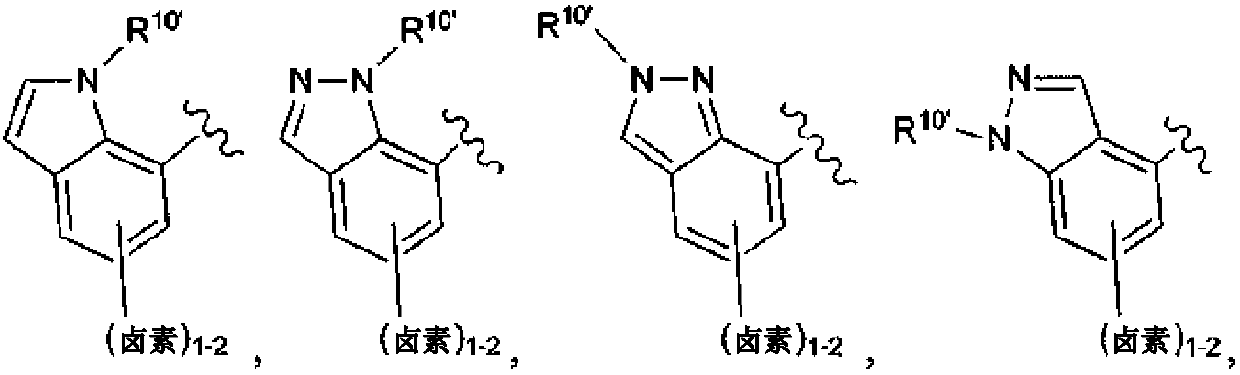
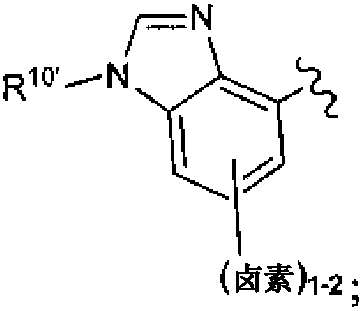
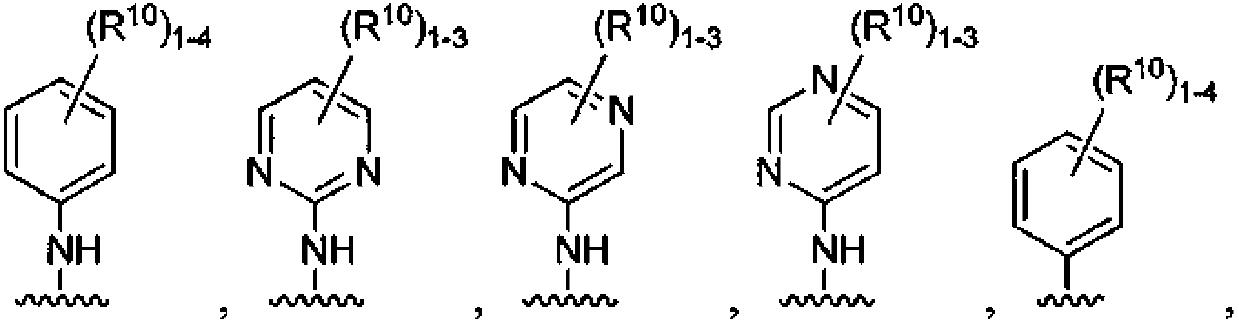
Macrocycles with hetrocyclic P2' groups as factor XIA inhibitors
A compound, heterocyclic group technology, applied in the field of novel macrocyclic compounds and their analogs, can solve problems such as limiting oral availability and intestinal permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0810] In another embodiment, the present invention provides a composition comprising at least one compound of the present invention, or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate thereof.
[0811] In another embodiment, the present invention provides a pharmaceutical composition comprising a pharmaceutically acceptable carrier and at least one compound of the present invention or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate thereof.
[0812] In another embodiment, the present invention provides a pharmaceutical composition comprising: a pharmaceutically acceptable carrier and a therapeutically effective amount of at least one compound of the present invention or a stereoisomer, tautomer, pharmaceutically acceptable salt or solvate thereof .
[0813] In another embodiment, the present invention provides methods for preparing compounds of the present invention.
[0814] In another embodiment, the present invention provides in...
Embodiment 30G
[1523] Example 30G. Preparation of (9R,13S)-13-amino-3-(difluoromethyl)-9-methyl-3,4,7,15-tetraazatricyclo[12.3.1.0 2,6 ] Octadeca-1(18),2(6),4,14,16-pentaen-8-one
[1524] 4N HCl (3.88 mL, 15.5 mmol) in dioxane was added to N-[(9R,13S)-3-(difluoromethyl)-9-methyl-8-oxo-3,4,7 ,15-Tetraazatricyclo[12.3.1.0 2,6 ]octadec-1(18),2(6),4,14,16-penten-13-yl]carbamate tert-butyl ester (2.25 g, 5.2 mmol) in MeOH (10 mL). The reaction mixture was stirred at rt for 2h. Cool the reaction mixture in an ice bath and add 7N NH in MeOH 3 (13.3 mL, 93.0 mmol). After 5min, use CH 2 Cl 2 (80 mL) the reaction mixture was diluted and the solid formed was filtered. The filtrate was concentrated to give (9R,13S)-13-amino-3-(difluoromethyl)-9-methyl-3,4,7,15-tetraazatricyclo[12.3.1.0 2,6 ] Octadeca-1(18),2(6),4,14,16-penten-8-one (1.3 g, 3.88 mmol, 75% yield). MS(ESI)m / z:336.3[M+H] + . 1 H NMR (400MHz, DMSO-d 6 )δ9.33(s,1H),8.71(d,J=5.0Hz,1H),7.94(t,J=58Hz,1H),7.85(s,1H),7.40(s,1H),7.32(d ...
Embodiment 45
[1721] Preparation of (9R,13S)-13-(4-{5-chloro-2-[(pyrimidin-2-yl)amino]phenyl}-6-oxo-1,6-dihydropyrimidin-1-yl) -3,9-Dimethyl-3,4,7,15-tetraazatricyclo[12.3.1.0 2,6 ] Octadeca-1(18),2(6),4,14,16-pentaen-8-one
[1722]
[1723] In a similar manner to the procedure described in Example 314, (9R,13S)- 13-(4-{5-chloro-2-[(pyrimidin-2-yl)amino]phenyl}-6-oxo-1,6-dihydropyrimidin-1-yl)-3,9-dimethyl Base-3,4,7,15-tetraazatricyclo[12.3.1.0 2 ,6 ] Octadeca-1(18),2(6),4,14,16-penten-8-one bistrifluoroacetate (2.75 mg, 19% yield). MS(ESI)m / z:582.5(M+H) + . 1 H NMR (400MHz, CD 3 OD)δ9.12(s,1H),8.74(d,J=5.1Hz,1H),8.46-8.42(m,3H),7.73(s,1H),7.66(d,J=2.6Hz,1H) ,7.54-7.48(m,2H),7.43(dd,J=8.9,2.5Hz,1H),6.85(t,J=5.0Hz,1H),6.79(s,1H),6.06(dd,J=12.7 ,4.3Hz,1H),4.05(s,3H),2.78-2.67(m,1H),2.42-2.31(m,1H),2.16-2.02(m,2H),1.69-1.44(m,2H), 1.02 (d, J=6.8Hz, 3H), 0.80-0.63 (m, 1H). Analytical HPLC (Method A): RT = 8.33 min, 97.9% purity; Factor XIa Ki = 2,000 nM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com