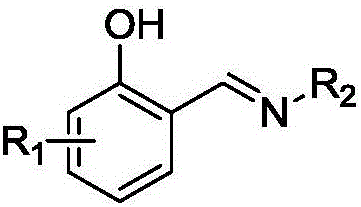
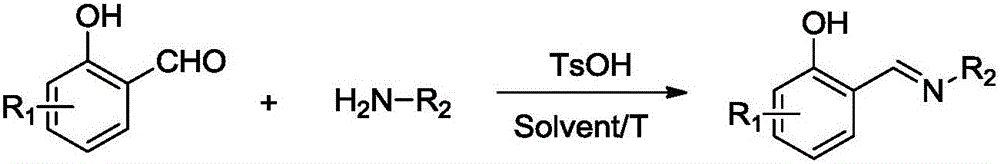
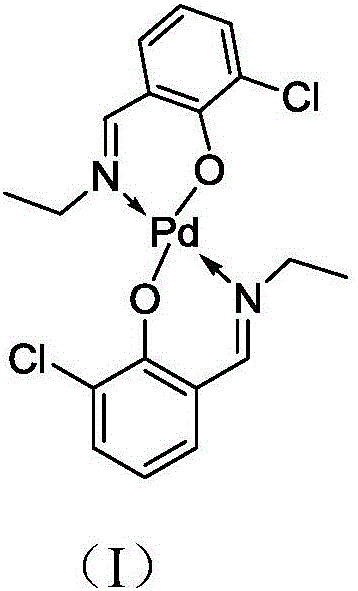
Salicyldenaminato schiff base metal complex catalyst as well as preparation method thereof and application thereof
A technology of salicylaldimine mat and metal complex, which is applied in the field of salicylaldimine Schiff base metal complex catalyst and its preparation, and can solve the problem of high polymer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Catalyst I
[0036] In the synthesis kettle, according to the formula weight ratio, dissolve 200 parts of 3-chloro-o-hydroxybenzaldehyde in 2000 parts of methanol and add 1 part of p-toluenesulfonic acid (TsOH) as a catalyst, and slowly add to the system at 60 ° C. 70 parts of ethylamine were added dropwise, and the reaction was maintained for 4h. After the reaction, methanol and excess ethylamine were evaporated at 80° C. under normal pressure, and the remaining product was recrystallized and purified with ethanol solvent / toluene.
[0037] The NMR and mass spectrometric analysis of the product is as follows: 1 H NMR (CDCl 3 ,400MHz):δ 8.54(1H,s),7.54-7.02(3H,m),5.35(1H,br s),3.59(2H,t,J=8.0Hz),1.18(3H,q,J=8.0 Hz); 13 C NMR (CDCl 3 ,100MHz):δ159.8,157.5,134.9,130.2,126.0,124.3,122.8,53.3,16.3; HRMS(ESI):Calcd for C 9 h 11 ClNO[M+H] + 184.0524, Found: 184.0529;
[0038] In the synthesis kettle, 38 parts of 2-chloro-N-ethyl salicylaldimine, 18 part...
Embodiment 2
[0041] Preparation of Catalyst II
[0042] In the synthesis kettle, according to the formula weight ratio, dissolve 250 parts of 2,4-di-tert-butylbenzaldehyde in 2000 parts of ethanol and add 2.5 parts of p-toluenesulfonic acid (TsOH) as a catalyst, and slowly 100 parts of aniline was added dropwise to the system, and the reaction was maintained for 6 hours. After the reaction, the ethanol was evaporated at 200pa and 40°C, and the remaining product was purified by recrystallization with methanol / toluene.
[0043] The NMR and mass spectrometric analysis of the product is as follows: 1 H NMR (CDCl 3 ,400MHz):δ 8.87(1H,s),7.52-7.06(7H,m),5.35(1H,br s),1.35(9H,s),1.34(9H,s); 13 C NMR (CDCl 3 ,100MHz):δ160.0,153.7,152.0,138.4,137.7,130.0,128.3,127.2,122.3,117.7,34.5,34.4,31.6,31.3; HRMS(ESI): Calcd for C 21 h 28 NO[M+H] + 310.2165, Found: 310.2164;
[0044] In the synthesis kettle, according to the formula weight ratio, 44 parts of 2,4-di-tert-butyl-N-phenyl salicylaldimi...
Embodiment 3
[0047] Preparation of Catalyst III
[0048] In the synthesis kettle, according to the formula weight ratio, 200 parts of 4-methoxy o-hydroxybenzaldehyde was dissolved in 2000 parts of methanol and 1 part of p-toluenesulfonic acid (TsOH) was added as a catalyst. 75 parts of ethylamine were added dropwise to the system, and the reaction was maintained for 4.5 hours. After the reaction, ethanol and ethylamine were evaporated at 200pa and 40°C, and the remaining product was purified by recrystallization with ethanol / toluene.
[0049] The NMR and mass spectrometric analysis of the product is as follows: 1 H NMR (CDCl 3 ,400MHz):δ 8.54(1H,s),7.71-6.48(3H,m),5.35(1H,br s),3.83(3H,s),3.59(2H,t,J=8.0Hz),1.18( 3H,q,J=8.0Hz); 13 C NMR (CDCl 3 ,100MHz): δ164.3, 162.1, 157.5, 133.4, 116.9, 107.0, 103.4, 55.8, 53.3, 16.3; HRMS (ESI): Calcd for C 10 h 14 NO 2 [M+H] + 180.1019, Found: 180.1022;
[0050] In the synthesis kettle, 36 parts of 3-methoxy-N-ethyl salicylaldimine, 18 parts...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com