Interferon lambda-containing aerosol inhalant
A technology of aerosol inhalation and interferon, which can be used in aerosol delivery, antiviral agents, respiratory diseases, etc., and can solve the problems of late discovery and reporting of type III interferon.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of interferon atomized inhalation
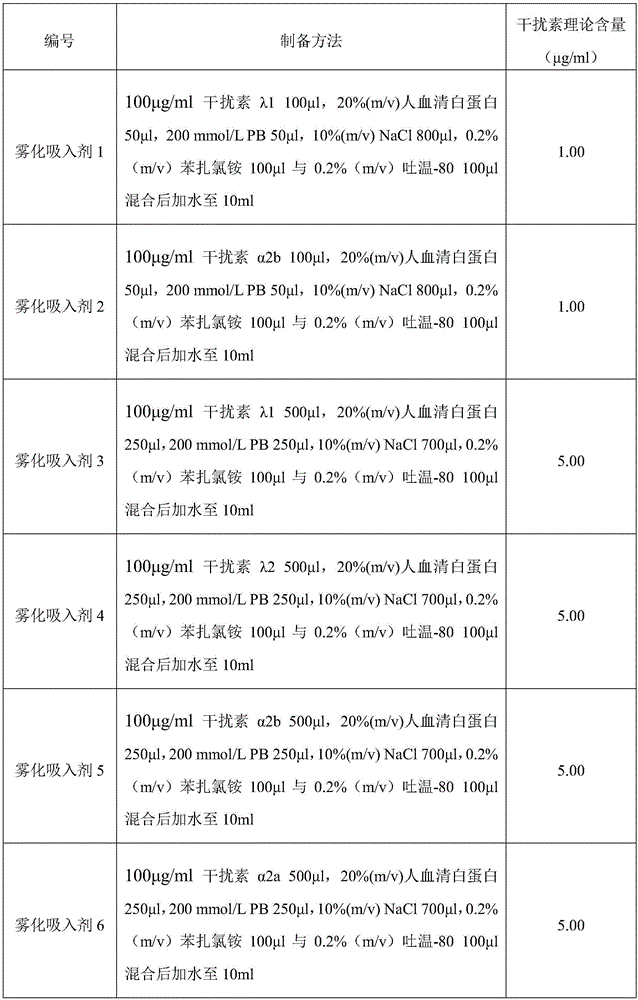
[0041] The nebulized inhalation of interferon was prepared according to the method in Table 1 below. Wherein "PB" is disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution (pH7.0).
[0042] Table 1 Preparation of interferon aerosol inhalation
[0043]
[0044]
[0045]
[0046]
Embodiment 2
[0047] Embodiment 2: the animal test of interferon atomized inhalation treatment viral pneumonia
[0048] 1) RSV virus culture
[0049] The RSV standard strain RSV-Long (quoted from the Beijing Institute of Pediatrics) was inoculated on Hep-2 cells, harvested when the cytopathic rate reached more than 80%, and the virus liquid was frozen in liquid nitrogen and stored at 37°C Melt, centrifuge at 1000rpm for 10 minutes, and take the supernatant for later use.
[0050] 2) Establishment of RSV mouse infection model
[0051] Take 126 6-7-week-old Balb / c mice, half male and half male, all anesthetized with ether, and instill 10 6 PFU / ml RSV virus liquid 0.1ml, instill the virus once a day, a total of 2 infusions, on the 3rd day, the mice showed positive reactions such as piloerection, restlessness, shortness of breath, and abdominal muscle convulsions, indicating that the RSV infection mouse model was successfully established.
[0052] 3) Grouping and administration
[0053] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com