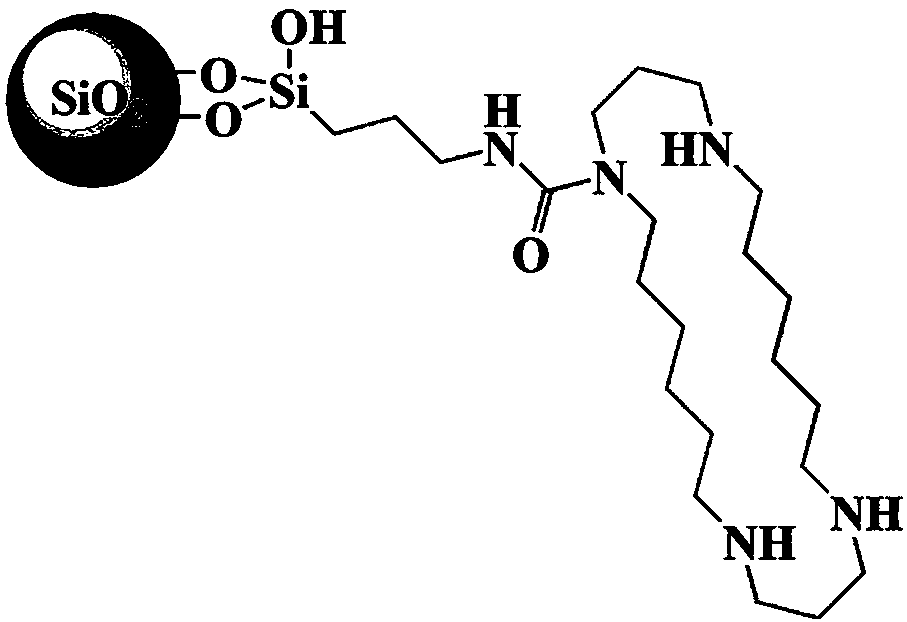
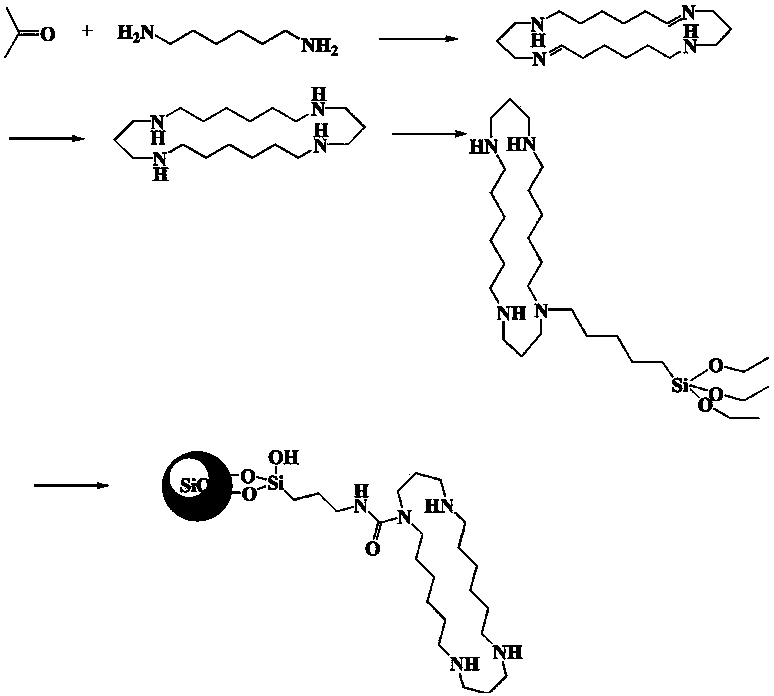
Tetraazadocosyl heterocyclic chromatographic stationary phase and its preparation method and application
A technology of docosyl and chromatographic stationary phase, which is applied in the field of synthesis of functional materials, can solve the problems of single type of target analyte, low degradation rate, strong toxicity, etc., and achieve simple chromatographic analysis conditions, simple preparation methods, and high separation efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Tetraazadocosyl Heterocyclic Chromatographic Stationary Phase
[0035] 1) 3g of silica gel was heated to reflux with concentrated hydrochloric acid for 4 hours, washed with water until neutral, and dried in vacuum at 120°C for 6 hours to obtain surface-activated silica gel.
[0036] 2) Dissolve 6 mmol of acrolein and equimolar hexamethylenediamine together in 50 ml of perchloric acid solution, stir well, and undergo a ring closure reaction to form a docoheterocyclic ligand.
[0037] 3) Weigh 6 mmol of docoheterocyclic ligand and dissolve it in 40 ml of tetrahydrofuran solution, mix with equimolar γ-isocyanatopropyltriethoxysilane in tetrahydrofuran (20 ml) solution, and let stand at room temperature for 30 minutes. Then, it was stirred for 12 hours, heated to 70°C, and refluxed for 2 hours to generate a docosane cyclosilane reagent.
[0038] 4) in N 2 Under ambient conditions, add 3 g of the acidified silica gel prepared in the first step to the docohet...
Embodiment 2
[0041] Preparation of Tetraazadocosyl Heterocyclic Chromatographic Stationary Phase
[0042] 1) 2.5g of silica gel was heated to reflux with concentrated hydrochloric acid for 4 hours, washed with water until neutral, and dried in vacuum at 120°C for 6 hours to obtain surface-activated silica gel.
[0043] 2) Dissolve 5 mmol of acrolein and equimolar hexamethylenediamine together in 40 ml of perchloric acid solution, stir well, and undergo a ring closure reaction to form a docoheterocyclic ligand.
[0044] 3) Weigh 5 mmol of docoheterocyclic ligand and dissolve in 30 ml of tetrahydrofuran solution, mix with equimolar γ-isocyanatopropyltriethoxysilane in tetrahydrofuran (15 ml) solution, and let stand at room temperature for 20 minutes. Then, it was stirred for 10 h, heated to 60° C., and refluxed for 1 h to generate a dococyclic silane reagent.
[0045] 4) in N 2 Under ambient conditions, add 2.5 g of the acidified silica gel prepared in the first step to the docosylcyclosil...
Embodiment 3
[0048] Preparation of Tetraazadocosyl Heterocyclic Chromatographic Stationary Phase
[0049] 1) 3.5g of silica gel was heated to reflux with concentrated hydrochloric acid for 6 hours, washed with water until neutral, and dried in vacuum at 120°C for 8 hours to obtain surface-activated silica gel.
[0050] 2) Dissolve 7 mmol of acrolein and equimolar hexamethylenediamine together in 60 ml of perchloric acid solution, stir well, and undergo a ring closure reaction to form a docoheterocyclic ligand.
[0051] 3) Weigh 7mmol of the docoheterocyclic ligand and dissolve it in 50ml of tetrahydrofuran solution, mix with an equimolar solution of γ-isocyanatopropyltriethoxysilane in tetrahydrofuran (25ml), and let stand at room temperature for 40 minutes. Then, it was stirred for 15 hours, heated to 80°C, and refluxed for 3 hours to generate a dococyclic silane reagent.
[0052] 4) in N 2 Under ambient conditions, add 3.5 g of the acidified silica gel prepared in the first step to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com