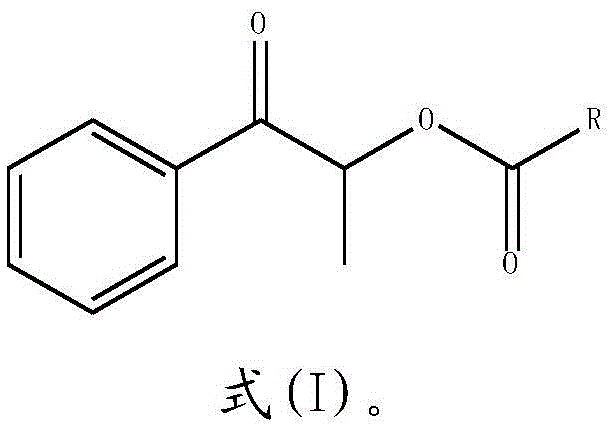
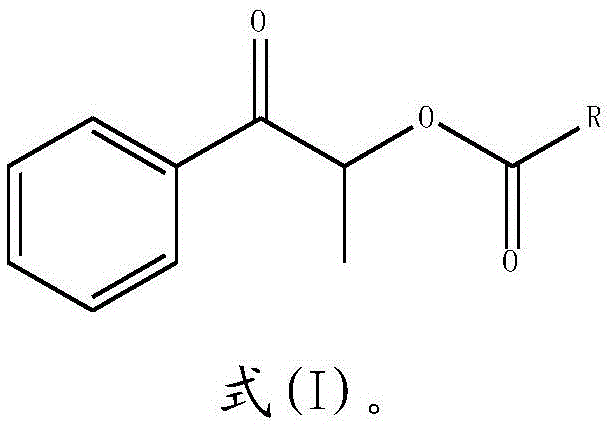
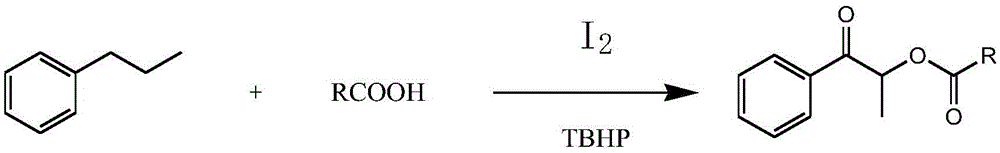Synthesizing method of propiophenone compound
A synthesis method and compound technology are applied in the synthesis field of propiophenone compounds, which can solve problems such as complicated reaction steps, and achieve the effects of reducing reaction cost, simplifying synthesis route and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The embodiment of the present invention provides a synthetic method of propiophenone compounds, comprising:
[0019] S1: Add propylbenzene, organic acid, iodine, and tert-butyl hydroperoxide into the reaction kettle, and react at 80°C-120°C for 8-24 hours.
[0020] In this step, the "one-pot method" can be used to synthesize propiophenone compounds. Specifically, propylbenzene, organic acid, tert-butyl hydroperoxide and iodine are added to the reaction kettle together, and heated at 80°C- React at 120°C for 8-24 hours to produce the target product. The specific reaction process is as follows:
[0021]
[0022] S2: After the reaction is completed, the reaction solution is extracted with an organic solvent, and dried to obtain a propiophenone compound:
[0023]
[0024] The embodiment of the present invention provides a kind of synthetic method of propiophenone compound, compared with prior art, the synthetic method provided by the present invention is to utilize p...
Embodiment 1
[0033] Add propylbenzene, acetic acid, iodine, and tert-butyl hydroperoxide with a mmol ratio of 1:1:0.2:6 into the reaction kettle, and react at 80°C for 8 hours; after the reaction is completed, use ethyl acetate The reaction solution was extracted and dried to obtain a colorless oily liquid that is Propiophenone Compound A:
[0034]
[0035] The above-mentioned colorless oily liquid is subjected to nuclear magnetic spectrum analysis, and the data are as follows:
[0036] 1 HNMR (CDCl 3 ,400MHz)δ1.56(d,J=6.8Hz,3H), 2.17(s,3H), 5.99(q,J=6.8Hz,1H), 7.49–7.54(m,2H), 7.60–7.63(m ,1H), 7.95–7.97(m,2H);
[0037] 13 CNMR (CDCl 3 ,100MHz) δ17.2, 20.5, 71.1, 128.2, 128.6, 133.7, 134.5, 170.4, 196.9;
[0038] After identification, the spectral data corresponded to the structural formula, proving that the synthesized compound was propiophenone compound A with a yield of 60%.
Embodiment 2
[0040] Add propylbenzene, isobutyric acid, iodine, and tert-butyl hydroperoxide with a mmol ratio of 1:2:0.3:8 into the reaction kettle, and react at 120°C for 24 hours; after the reaction is completed, use acetic acid The reaction solution was extracted with ethyl ester and dried to obtain a colorless oily liquid, i.e. propiophenone compound B:
[0041]
[0042] Carry out nuclear magnetic spectrum analysis to above-mentioned colorless oily liquid, the data are as follows:
[0043] 1 HNMR (CDCl 3,300MHz) δ1.19-1.20(m,6H), 1.55(q,J=6.9Hz,3H), 2.65(hept,J=6.9Hz,1H), 5.97(q,J=6.9Hz,1H), 7.44-7.53(m,2H), 7.58-7.63(m,1H), 7.93-7.98(m,2H);
[0044] 13 CNMR (CDCl 3 ,75MHz) δ17.1, 18.7, 18.9, 33.9, 71.1, 128.4, 128.7, 133.6, 134.5, 176.8, 197.3;
[0045] After identification, the spectral data corresponded to the structural formula, proving that the synthesized compound was propiophenone compound B with a yield of 69%.
[0046] As can be seen from the foregoing examples, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com