Preparation method of rhodium compounds
A rhodium compound and compound technology, applied in the field of preparation of rhodium compounds, can solve problems such as unfavorable production and low yield, and achieve the effects of simple operation, high purity and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
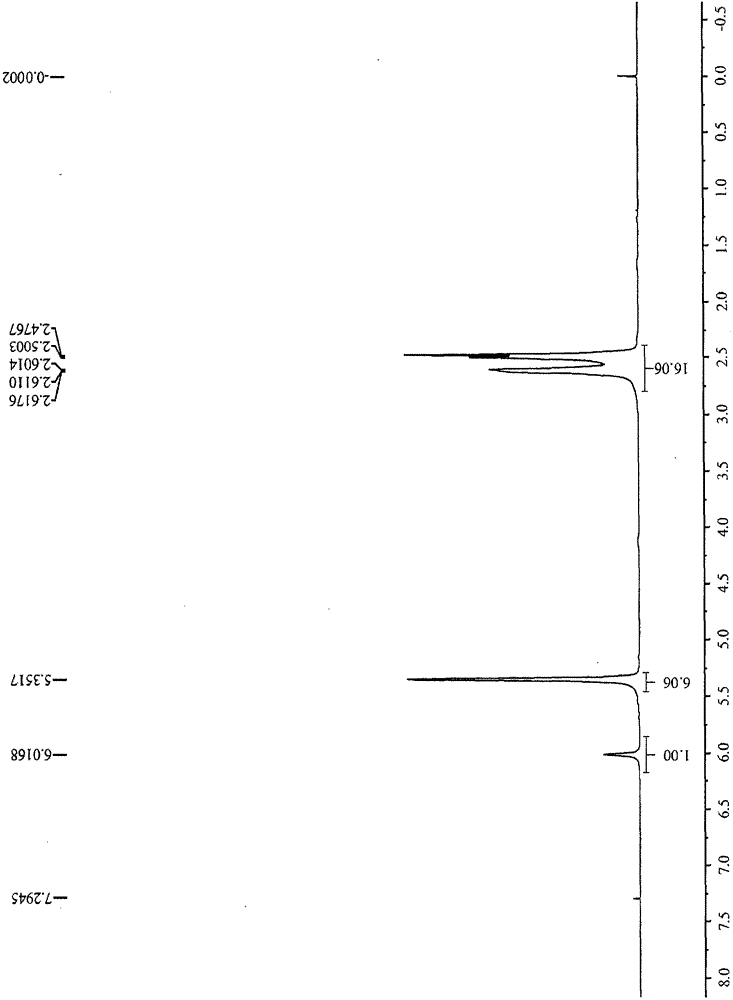
[0026] Dissolve 10 grams of rhodium trichloride trihydrate in 100 milliliters of absolute ethanol and 20 milliliters of water, measure 37.4 milliliters of 1,5-cyclooctadiene in a constant pressure funnel, and replace it with nitrogen for protection, and start to drop 1,5-cyclooctadiene Octadiene, after the dropwise addition, the temperature was raised to 82°C and kept stirring for 18 hours. The reaction solution was concentrated by rotary evaporation, cooled and filtered to obtain 1,5-cyclooctadiene rhodium chloride dimer. Then all the above solids were dissolved in 240 milliliters of dichloromethane and 17.7 milliliters of 1,5-cyclooctadiene, under nitrogen protection, slowly added dropwise at 20°C 60 milliliters of acetone solution containing 7.5 grams of silver tetrafluoroborate, dropwise After heating up to 35°C for reaction for 24 hours, filter, concentrate by rotary evaporation, filter, rinse with methyl tert-butyl ether, and dry under vacuum at room temperature to obtai...
Embodiment 2
[0028] Dissolve 100 grams of rhodium trichloride trihydrate in 1000 milliliters of absolute ethanol and 200 milliliters of water, measure 370 milliliters of 1,5-cyclooctadiene in a constant pressure funnel, and replace it with nitrogen to protect it, and start to add 1,5-cyclooctadiene dropwise. After the addition of octadiene, the temperature was raised to 82°C and kept stirring for 24 hours, concentrated by rotary evaporation, cooled and filtered to obtain 1,5-cyclooctadiene rhodium chloride dimer. It is all dissolved in 2400 milliliters of methylene chloride and 160 milliliters of 1,5 cyclooctadiene, under nitrogen protection, slowly add dropwise 600 milliliters of acetone solution containing 75 grams of silver tetrafluoroborate at 20 ° C, in Reflux at 45°C for 36 hours, filter, concentrate by rotary evaporation, filter, rinse with methyl tert-butyl ether, and dry under vacuum at room temperature to obtain 141.05 g of bis-1,5-cyclooctadiene rhodium tetrafluoroborate. The me...
Embodiment 3
[0030] Dissolve 100 grams of rhodium trichloride trihydrate in 1000 milliliters of absolute ethanol and 200 milliliters of water, measure 374 milliliters of 1,5-cyclooctadiene in a constant pressure funnel, and start to drop 1,5-cyclooctadiene under nitrogen protection. After the addition of octadiene, the temperature was raised to 82°C and kept stirring for 24 hours, concentrated by rotary evaporation, and filtered to obtain 1,5-cyclooctadiene rhodium chloride dimer. Then it was all dissolved in 2000 milliliters of methylene chloride and 160 milliliters of 1,5 cyclooctadiene, under the protection of nitrogen, slowly added dropwise at 20 ℃ containing 600 milliliters of acetone solution containing 75 grams of silver tetrafluoroborate, in Reflux at 45°C for 30 hours, filter, concentrate by rotary evaporation, filter, rinse with methyl tert-butyl ether, and dry under vacuum at room temperature to obtain 138 g of bis-1,5-cyclooctadiene rhodium tetrafluoroborate. The measured metal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com