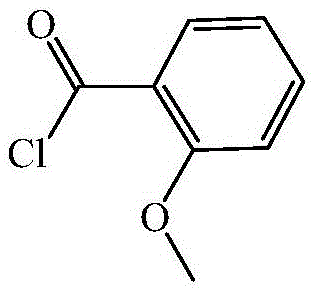
Preparing method for o-methoxybenzoyl chloride
A technology of o-methoxybenzoyl chloride and methoxybenzoyl chloride, applied in the field of preparation of o-methoxybenzoyl chloride, can solve the problems of inconvenient industrial production, unfavorable environmental protection, long reaction time, etc. The effect of mild operation, mild reaction, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 15.23g (0.1mol) of o-methoxybenzoic acid, 30.5g of dichloroethane, and 0.3g (0.004mol) of DMF into a reaction vessel with a tail gas absorption device, raise the temperature to 40°C, and slowly add the concentration 2mol / L BTC-C 2 h 4 Cl 2 The solution was 15.5ml, and after the dropwise addition, the reaction was refluxed for 1 hour. After the solvent was recovered by distillation under reduced pressure, 16.88g of o-methoxybenzoyl chloride was obtained, with a content of 99.62% (gas chromatography), and a yield of 98.57%.
Embodiment 2
[0018] Add 15.23g (0.1mol) of o-methoxybenzoic acid, 45.7g of dichloroethane as a solvent, and 0.48g (0.006mol) of pyridine into a reaction vessel with a tail gas absorption device, raise the temperature to 42°C, and slowly add the concentration 2mol / L of BTC / C 2 h 4 Cl 2 The solution was 15.5ml, and after the dropwise addition, the reaction was refluxed for 1.5 hours. After the solvent was recovered by distillation under reduced pressure, 16.86g of o-methoxybenzoyl chloride was obtained, with a content of 99.68% (gas chromatography), and a yield of 98.28%.
Embodiment 3
[0020] Add 15.23g (0.1mol) of o-methoxybenzoic acid, 60.9g of dichloroethane, and 0.38g (0.005mol) of DMF into a reaction vessel with a tail gas absorption device, raise the temperature to 45°C, and slowly add the concentration 2mol / L of BTC / C 2 h 4 Cl 2 The solution was 15.5ml, and after the dropwise addition, the reaction was refluxed for 2 hours. After the solvent was recovered by distillation under reduced pressure, 16.92g of o-methoxybenzoyl chloride was obtained, with a content of 99.87% (gas chromatography), and a yield of 98.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com