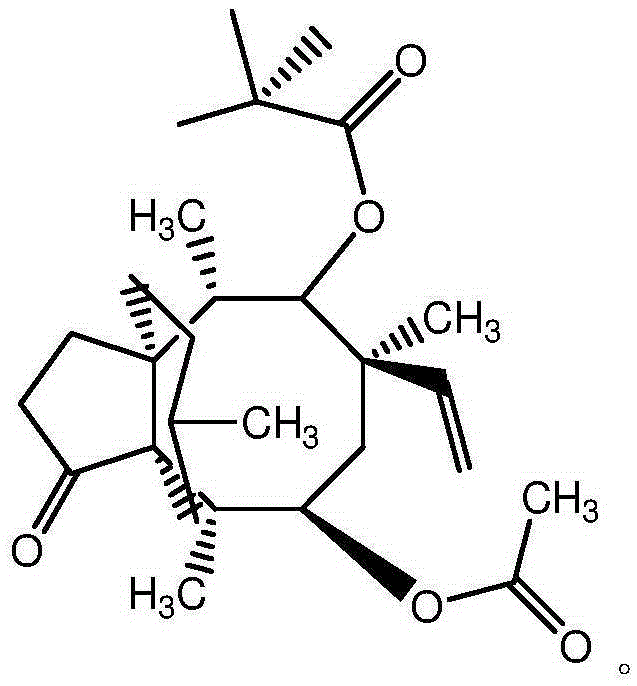
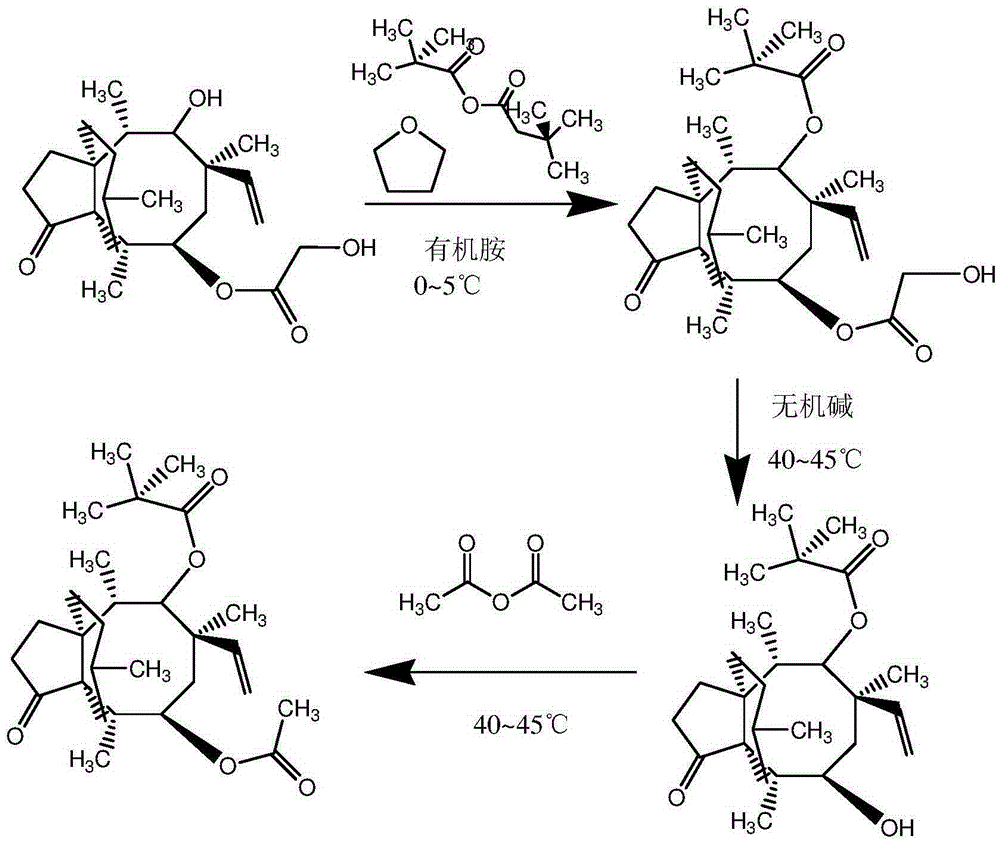
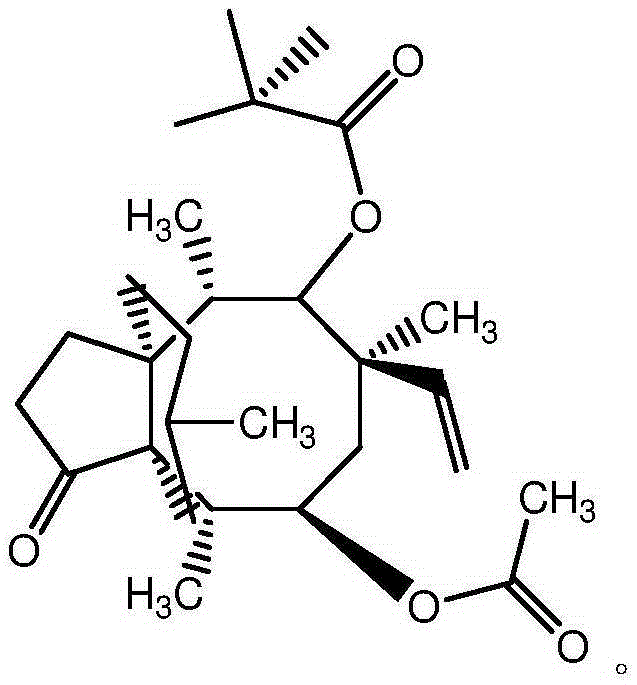
Synthetic intermediate of pleuromutilin antibiotics and synthetic method thereof
A technology for pleuromutilin and a synthesis method, which is applied to the synthetic intermediates of pleuromutilin antibiotics and the field of synthesis thereof, can solve the problems of destruction selectivity, the inability of mtriptyline to be used for the synthesis of antibiotics, etc., and achieves cost controllable Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Take 1000kg of pleuromutilin crystals, put them into a 10m3 glass-lined reactor, add 4000kg of pyridine, stir to dissolve, cool down to 0°C, put in 300kg of di-tert-butyl dicarbonate and 50kg of tetrahydrofuran, and keep warm for 3 hours. After the reaction was completed, it was concentrated and distilled under reduced pressure until a dry solid was obtained. Add 4000kg of water to the tank, adjust the pH to 10.5 with 20% sodium hydroxide, heat up to 40°C, hydrolyze for 20 hours, add 2000kg of methyl isobutyl ketone according to the ratio of water: methyl isobutyl ketone = 2:1 base ketone, keep warm at 40°C for extraction, and keep the organic phase after the extraction is completed. In the state of heat preservation, slowly add 250kg of acetic anhydride dropwise to the organic phase, and keep the reaction for 3 hours to obtain a methyl isobutyl ketone solution of double-protected mutriptyline, with a content of 37.68% and a yield of 90.44%, which can be directly used f...
Embodiment 2
[0023] Take 200kg of pleuromutilin crystals, put them into a 6m3 glass-lined reactor, add 900kg of piperidine, stir to dissolve, cool down to 5°C, put in 50kg of di-tert-butyl dicarbonate and 12kg of tetrahydrofuran, and keep warm for 4 hours. After the reaction was completed, the mixture was concentrated by raising the temperature and distilled under reduced pressure until a dry solid was obtained. Add 1000kg of water to the tank, adjust the pH to 11.0 with 15% potassium hydroxide, heat up to 42°C, hydrolyze for 22 hours, add 400kg of methyl isobutyl ketone according to the ratio of water:methyl isobutyl ketone=2.5:1 Ketones were kept at 42°C for extraction, and the organic phase was retained after the extraction was completed. In the state of heat preservation, 40 kg of acetic anhydride was slowly added dropwise to the organic phase, and the heat preservation reaction was carried out for 4 hours to obtain a methyl isobutyl ketone solution of double-protected mutriptyline wit...
Embodiment 3
[0025] Take 100g of pleuromutilin crystals, put them into a 1000L three-necked flask, add 500g of diethylamine, stir to dissolve, cool down to 2°C, put in 25g of di-tert-butyl dicarbonate and 7.5g of tetrahydrofuran, and keep warm for 5 hours. After the reaction was completed, the mixture was concentrated by raising the temperature and distilled under reduced pressure until a dry solid was obtained. Add 450g of water to the tank, adjust the pH to 10.7 with 20% ethanol / sodium ethylate, heat up to 44°C, hydrolyze for 21 hours, add 150g of methyl isobutyl ketone according to the ratio of water: methyl isobutyl ketone = 3:1 Butyl ketone, keep warm at 44°C for extraction, keep the organic phase after the extraction is completed. In the state of heat preservation, 30 g of acetic anhydride was slowly added dropwise to the organic phase, and the heat preservation reaction was carried out for 5 hours to obtain a methyl isobutyl ketone solution of double-protected mutriptyline with a co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com