Method for detecting secondary amine with thiobarbituric acid derivatives serving as probe molecules and preparation of thiobarbituric acid derivatives for detecting secondary amine
A technology of thiobarbituric acid and probe molecules, which is applied in chemical instruments and methods, analysis through chemical reactions of materials, measurement of color/spectral characteristics, etc., to achieve good selectivity, stable chemical properties, and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Preparation of probe molecule 1,3-diphenyl 5 (2-furyl methylene) thiobarbituric acid (DPFTBA)
[0048] Aniline (11mmol), triethylamine (36.2mmol) and tetrahydrofuran 10mL were placed in a flask, stirred in an ice bath for 10min, and then carbon disulfide (11mmol) was added dropwise to the mixture within 30min; The solution was stirred and reacted at room temperature for 10 h; then cooled in an ice bath, TsCl (12.1 mmol) was slowly added, and stirred for 1 h to obtain 0.965 g of phenyl isothiocyanate. Dissolve 0.965g of phenyl isothiocyanate in dichloromethane, add 0.665g of aniline under ice-cooling condition, and stir for 45min. After the reaction is complete, filter; wash the filter cake several times with dichloromethane, and dry to obtain 1.497 g of N,N-diphenylthiourea. Mix 1.497g N,N-diphenylthiourea and dichloromethane in a round bottom flask, then add 7mmol malonyl chloride, heat to reflux for 1h, after the reaction is complete, remove the solvent...
Embodiment 2
[0051] Embodiment 2: Preparation of probe molecule 1,3-diethyl-5-(2-furyl methylene) thiobarbituric acid (DEFTBA)
[0052] Using steps similar to Example 1, 1,3-diethyl-2-thiobarbituric acid was obtained, which was mixed with ethanol and stirred evenly, and excess furan aldehyde and 0.5 mL pyridine were added, and stirred at room temperature for 2-3 h, Filtration, column chromatography separation, obtain target product 1,3-diethyl-5-(2-furyl methylene) thiobarbituric acid (1,3-diethyl-5-(2-furan-methylene )thiobarbituricacid) (structure shown in formula 6) 0.56g.
[0053] 1 HNMR (400MHz, CDCl 3 ): δppm = 8.73 (d, J = 3.9Hz, 1H), 8.46 (s, 1H), 7.91 (d, J = 1.2Hz, 1H), 6.78 (ddd, J = 3.8, 1.5, 0.7Hz, 1H) , 4.59 (qd, J=7.0, 4.6Hz, 4H), 1.33 (q, J=6.9Hz, 6H). (NMR image see Figure 10 )
[0054]
Embodiment 3
[0055] Embodiment 3: the application of the probe that detects secondary amines
[0056] The molecular probe (abbreviation) in Example 1 was dissolved in a mixed solvent of methanol containing 2% DMSO to prepare a probe solution with a concentration of 0.1 mg / mL. Add it into different solutions to be tested, shake it well at room temperature, let it stand in the dark for 0.5-1.0 h, and collect data with an ultraviolet-visible spectrophotometer.
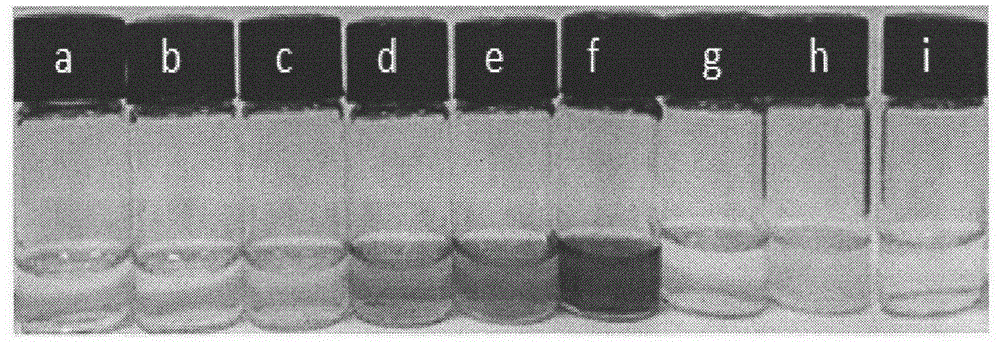
[0057] figure 1 The colorimetric physical map shows the selectivity of its secondary amine compounds.
[0058] Figure 2 to Figure 5 The spectral properties of the probe molecule, the relationship between the concentration of the secondary amine and the absorbance, etc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com