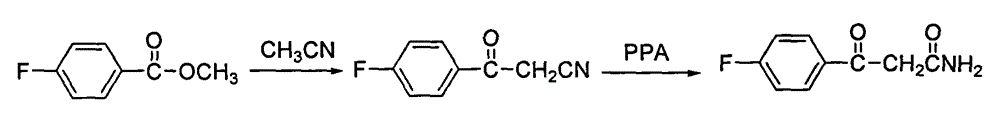
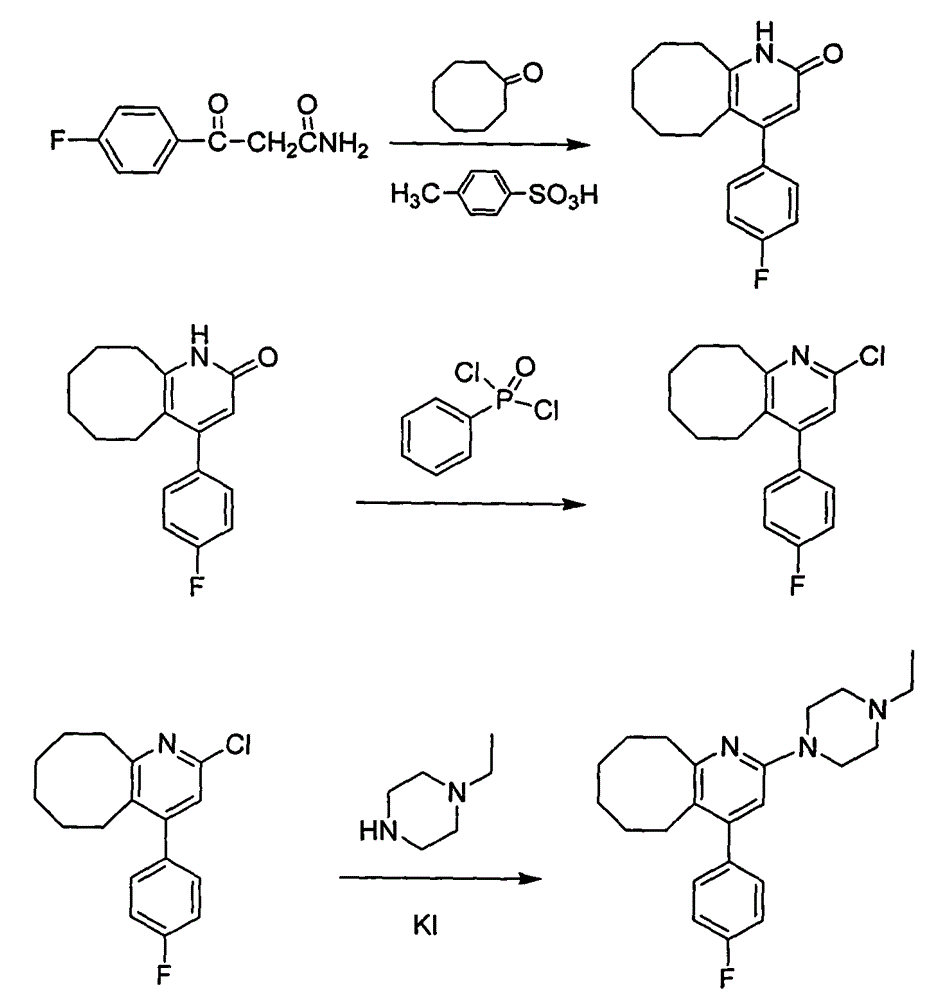
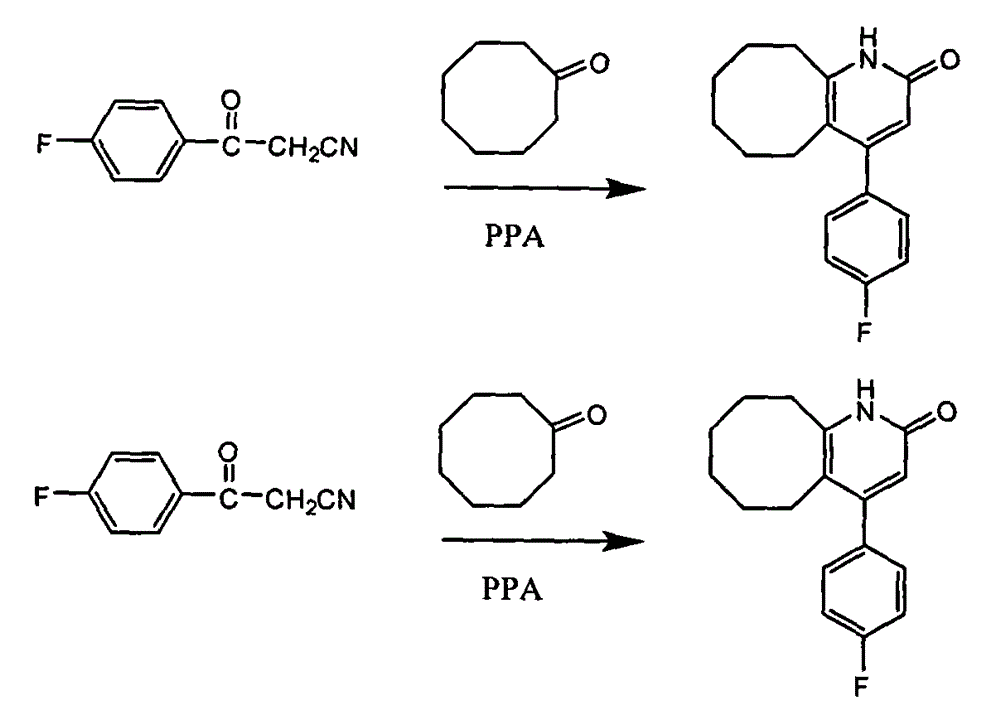
Method for preparing high-purity blonanserin
A blonanserin and high-purity technology, applied in organic chemistry and other fields, can solve problems such as long reaction routes, high costs, and gaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 15kg polyphosphoric acid and 6750g phosphoric acid into the reactor, start stirring, the system is heated up to 55-60°C, add 1500g p-fluorobenzoylacetonitrile in batches, continue stirring for 10min, add 667g cyclooctanone in batches, and keep warm for 30min , heating to raise the temperature of the system to 105-110°C, follow the reaction, after 2.5h, add 167.5g cyclooctanone, continue the reaction for about 1.5h, add 167.5g cyclooctanone again, follow the reaction until the reaction of the raw materials is complete , cool the reaction system and keep the temperature between 80 and 90°C, slowly add 13.5 LDMF dropwise, after the drop is complete, pour the reaction solution into 300L water, stir, after 30min, add 1L of petroleum ether, a yellow solid precipitates, continue to stir for 1h , filtered, the filter cake was washed with petroleum ether, and dried to obtain 900.5g of solid, which was refined by adding 1L of isopropanol to obtain 868.6g of yellow solid, with ...
Embodiment 2
[0032] Add 15kg polyphosphoric acid and 6750g phosphoric acid into the reactor, start stirring, the system is heated up to 55-60°C, add 1500g p-fluorobenzoylacetonitrile in batches, continue stirring for 10min, add 667g cyclooctanone in batches, and keep warm for 30min , heating to raise the temperature of the system to 115-120°C, follow the reaction, after 2.5h, add 167.5g cyclooctanone, continue the reaction for about 1.5h, add 167.5g cyclooctanone again, follow the reaction until the reaction of the raw materials is complete , cool the reaction system and keep the temperature between 80 and 90°C, slowly add 13.5 LDMF dropwise, after the drop is complete, pour the reaction solution into 300L water, stir, after 30min, add 1L of petroleum ether, a yellow solid precipitates, continue to stir for 1h , filtered, the filter cake was washed with petroleum ether, and dried to obtain 853.8g of solid, which was refined by adding 1L of isopropanol to obtain 821.3g of yellow solid, with ...
Embodiment 3
[0034] Add 15kg polyphosphoric acid and 6750g phosphoric acid into the reactor, start stirring, the system is heated up to 55-60°C, add 1500g p-fluorobenzoylacetonitrile in batches, continue stirring for 10min, add 667g cyclooctanone in batches, and keep warm for 30min. Heating to raise the temperature of the system to 105-110°C, follow the reaction, after 2.5 hours, add 167.5g cyclooctanone, continue the reaction for about 1.5h, add 167.5g cyclooctanone again, follow the reaction until the reaction of the raw materials is complete, Cool the reaction system and keep the temperature between 80 and 90°C, slowly add 13.5L of ethyl acetate dropwise, after the drop is complete, pour the reaction solution into 300L of water, stir, after 30min, add 1L of petroleum ether, a yellow solid precipitates, continue Stir for 1 h, filter, wash the filter cake with petroleum ether, and dry to obtain 832.4 g of solid, add 1 L of isopropanol to refine, obtain 802.1 g of yellow solid, which is the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com