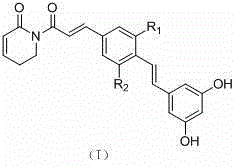
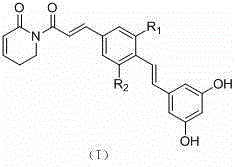
Application of resveratrol-group piperlongumine analogue in medicine
A technology based on peronamide and resveratrol, which is applied in the field of application of resveratrol-based peronamide analogs in the treatment of malignant tumors, and can solve the problem of unseen applications of resveratrol-based peronamide analogs And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: Compound BLBB-1 preparation of
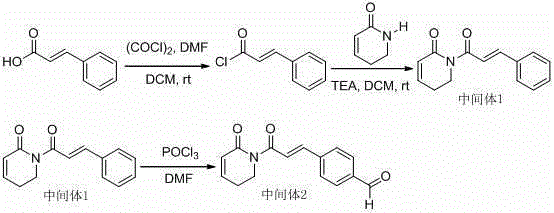
[0018] The synthetic route is as follows:
[0019]
[0020] For the synthetic route of intermediate 1, please refer to the reference (Shoujiao Pengetal, J. Med. Chem. 2015, 58, 5242? 5255).
[0021] For the synthetic route of intermediate 2, please refer to the reference (Liu Wenhu et al., Acta Pharmaceutica Sinica, 2014, 49(2):217-224).
[0022]
[0023] The synthetic route of intermediate 3 can be found in references (Hou Jian et al., China Journal of Pharmaceutical Industry, 2008, 39, 1, 1-8).
[0024] The synthetic route of compound BLBB-1 is shown in the literature (Hou Jian et al., China Journal of Pharmaceutical Industry, 2008, 39, 1, 1-8):
[0025]
[0026] Dissolve the intermediate 310mmol in 10ml DMF under ice bath, add NaOCH 3 10mmol and vigorously stirred, then dropwise into the intermediate 210mmol, room temperature for 10h. Then it was poured into ice water, extracted with ethyl acetate, washed wit...
Embodiment 2
[0030] Embodiment 2: compound BLBB-2 preparation of
[0031] The synthetic route is as compound BLBB-1.
[0032] 1 HNMR (400MHz, DMSO): δ9.50(s, 1H), 9.14(s, 1H), 7.92(d, J=15.6Hz, 1H), 7.34(d, J=15.6Hz, 1H), 6.94(d ,J=16.4Hz, 1H), 6.92-6.90(m, 3H), 6.82(d, J=16.4Hz, 1H), 6.52(d, J=2.0Hz, 2H), 6.21(t, J=2.4Hz , 1H), 6.01(t, J=9.6Hz, 1H), 4.00(t, J=6.4Hz, 2H), 3.88(s, 6H), 2.44(m, 2H).
[0033] MS-ESI (m / z): 421.15.
Embodiment 3
[0034] Embodiment 3: compound BLBB-3 preparation of
[0035] The synthetic route is as compound BLBB-1.
[0036] 1 HNMR (400MHz, DMSO): δ9.52(s, 1H), 9.12(s, 1H), 7.90(d, J=15.6Hz, 1H), 7.36(d, J=15.6Hz, 1H), 6.98(d ,J=16.4Hz,1H),6.94-6.92(m,3H),6.80(d,J=16.4Hz,1H),6.54(d,J=2.0Hz,2H),6.22(t,J=2.4Hz , 1H), 6.03(t, J=9.6Hz, 1H), 4.00(t, J=6.4Hz, 2H), 2.42(m, 2H).
[0037] MS-ESI (m / z): 397.11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com