A kind of synthetic method containing camphenyl schiff base compound
A synthesis method and compound technology are applied in the field of synthesis of camphenyl-containing Schiff base compounds, can solve the problems such as there is no synthetic method for camphenyl-containing Schiff base compounds, and achieve high yield, little secondary pollution, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
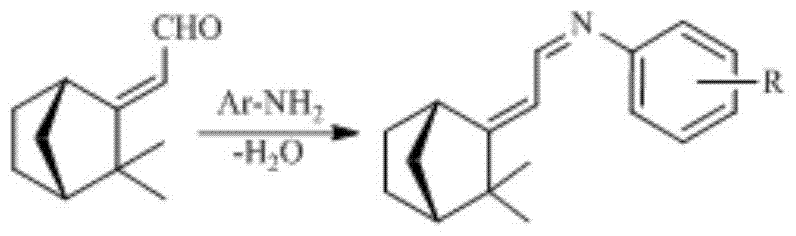
[0019] A kind of synthetic method containing camphenyl Schiff base compound, is characterized in that, concrete synthesis step is as follows:
[0020] (1) take camphenal as raw material, add aromatic amine compound in the reaction device with water separator, aromatic amine is aniline or substituted aniline, and substituted aniline is o-toluidine, p-toluidine, p-bromoaniline, o-toluidine A kind of chloroaniline, p-chloroaniline, p-methoxyaniline, m-nitroaniline, p-nitroaniline;
[0021] The molar ratio of camphenal and aromatic amine is 1: 1~1.1 for feeding, then adding organic solvent benzene or cyclohexane, stirring and refluxing for 2~3h, and tracking the reaction progress in gas phase until the content of the target product no longer increases;
[0022] (2) benzene or cycloethane solvent in the above-mentioned reaction solution are steamed, and the thick product obtained is purified with methanol or ethanol, and then dried to obtain camphenyl Schiff base compounds, and its...
Embodiment 1
[0025] Embodiment 1: General method of synthesis of camphenyl-containing Schiff base compounds (aniline and p-chloroaniline)
[0026] (1) Add 0.05mol of camphenal, 0.055mol of p-chloroaniline and 30ml of cyclohexane in a 150ml conical flask equipped with a water separator, add water-carrying agent in the water separator, stir and reflux for 2h , use the gas phase to track the reaction process until the product content no longer increases;
[0027] (2) The cyclohexane in the reactant is evaporated, and the target products, camphenal aniline Schiff base and camphenal o-toluidine Schiff base, are distilled off under reduced pressure.
[0028] The product is as follows:
[0029] ①Camhenal aniline Schiff base, molecular formula: C 17 H 21 N, molecular weight: 239.17, brown liquid, b.p.=165~167℃ / 200Pa, purity 95.65%, yield 73%, the attribution of structural analysis spectrum data is as follows:
[0030] FT-IR,ν max (cm -1 ): 2961, 2919, 2868 (C-H), 1648 (C=N), 1602, 1586, 1484...
Embodiment 2
[0039] Embodiment 2: General method for synthesis of camphenyl Schiff base compounds (o-toluidine, p-toluidine, p-bromoaniline, o-chloroaniline, p-methoxyaniline, m-nitroaniline, p-nitroaniline)
[0040] (1) Add 0.05mol of camphenal, 0.055mol of aromatic amine and 30ml of cyclohexane in a 150ml conical flask equipped with a water separator, add water-carrying agent in the water separator, stir and reflux for 2h, Follow the progress of the reaction with the gas phase until the product content no longer increases;
[0041] (2) The cyclohexane in the reactant was steamed, ethanol was added, placed to allow natural crystallization, after the crystals were filtered out, recrystallized in ethanol, filtered under reduced pressure, the solid was rinsed with cold ether, and dried in vacuo , to obtain a solid powdery camphenal acetal substituted aniline Schiff base.
[0042] The product is as follows:
[0043] ① camphenal acetal p-toluidine Schiff base, molecular formula: C 18 H 23 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com