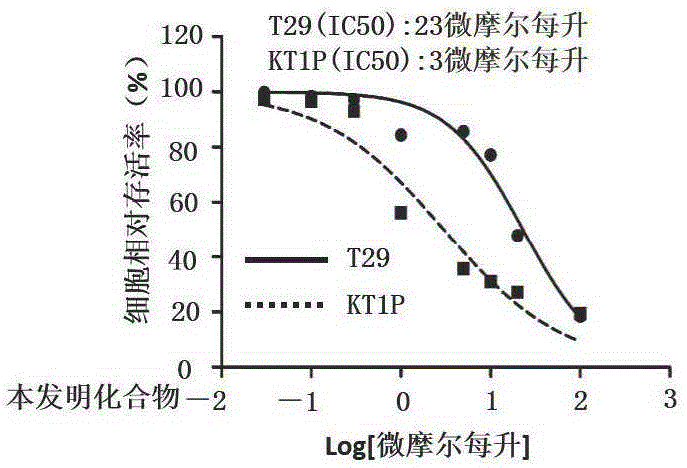
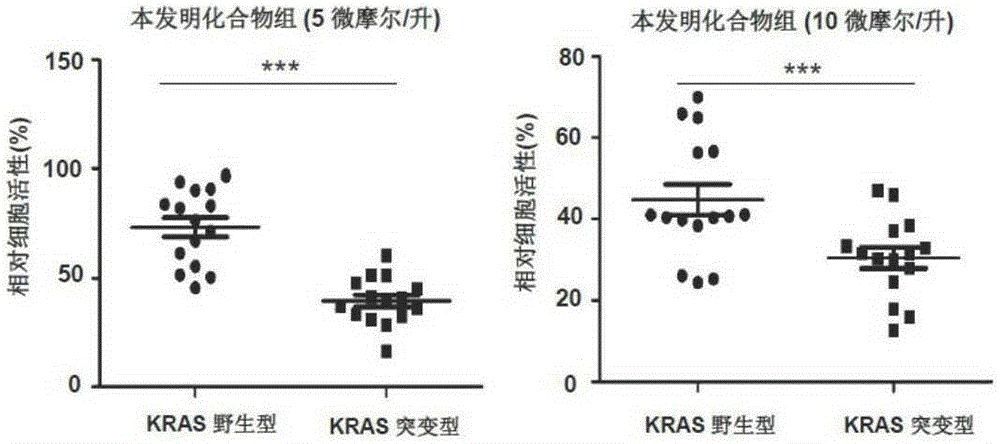
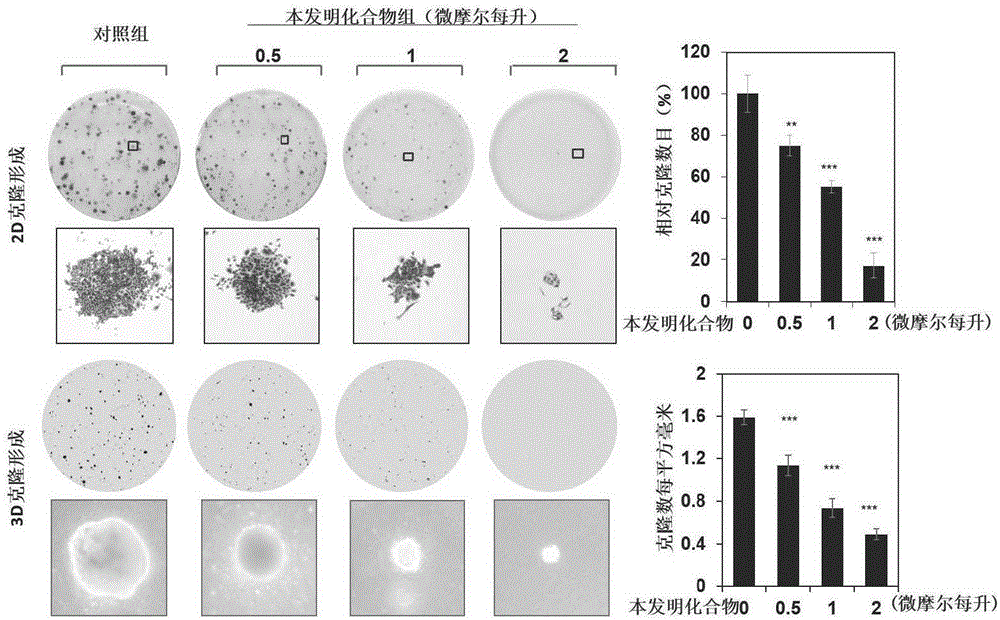
Benzotriazole compound, preparation method and medical application thereof
A technology of benzotriazole and benzotriazole, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Embodiment one: the preparation of each compound
Embodiment 1-1
[0118] Example 1-1, 5-bromofuran-2-carboxylic acid [2-(4-methoxyphenyl)-2H-benzotriazol-5-yl]-amide (RS001)
[0119] Dissolve p-methoxyaniline (2.48g, 20.14mmol) in 48ml of aqueous hydrochloric acid solution (4M), add sodium nitrite (1.67g, 24.17mmol) under ice-bath conditions, and react for half an hour, then add sulfamic acid Ammonium (3.16g) was slowly added to the system at 0°C and stirring was continued for half an hour. The pH of the solution was adjusted to 5-6 with sodium acetate, and o-phenylenediamine hydrochloride (3.33 g, 18.42 mmol) was added to the reaction system under ice-bath conditions, and stirred overnight. After the completion of the reaction as detected by TLC, adjust the pH to greater than 14 with sodium hydroxide solution, extract with ethyl acetate and water, combine the organic phases, and distill under reduced pressure to obtain a dry crude azo compound. The azo compound was dissolved with 40ml of pyridine, and 10g of anhydrous copper sulfate was ad...
Embodiment 1-2
[0129] Example 1-2, Preparation of 5-bromofuran-2-carboxylic acid [2-(4-chlorophenyl)-2H-benzotriazol-5-yl]-amide (RS002)
[0130] Substitute p-methoxyaniline with 4-chloroaniline, and prepare RS002 according to the method for preparing compound RS001. 1 HNMR(DMSO,300MHz):δ10.51(s,1H),8.49(s,1H),8.27(d,J=8.7Hz,2H),8.98(dd,J=0.3,0.3Hz,1H),7.78 (t, J=0.9Hz, 1H), 7.71(s, 2H), 7.44(d, J=3.3Hz, 1H), 6.87(d, J=3.3Hz, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com