(R)-1-benzyl-3-methyl-1,2,3,6-tetrahydropiperidyl synthetic method
A technology of tetrahydropiperidine and a synthetic method, applied in directions such as organic chemistry, to achieve the effects of saving production costs, simple post-processing, and shortening time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
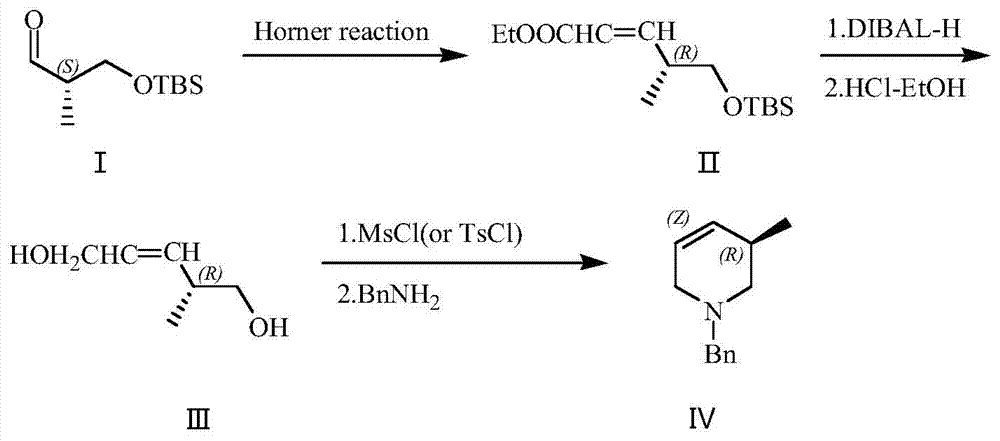
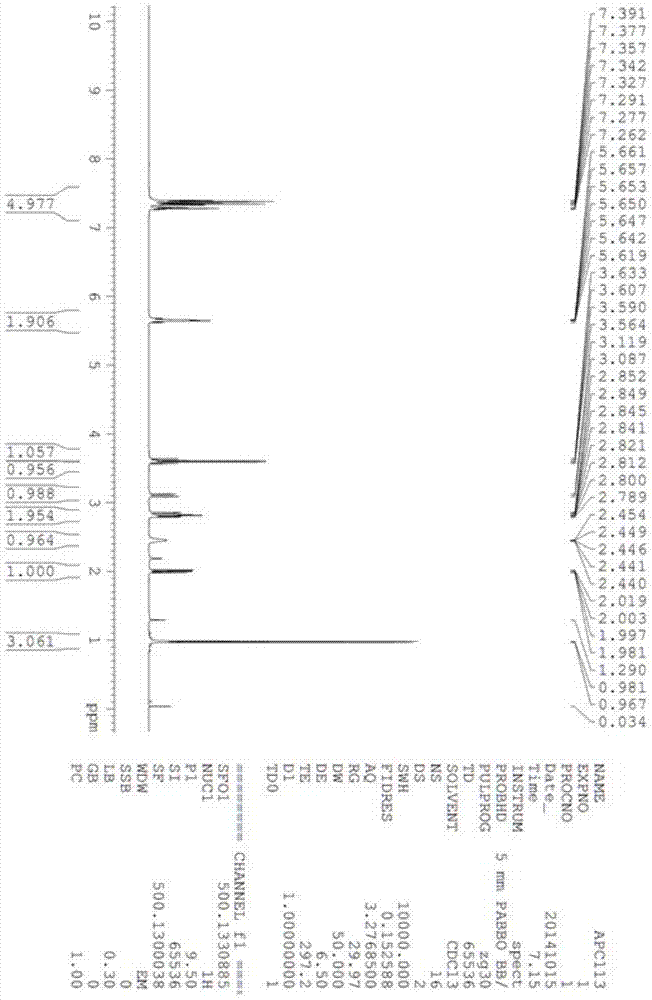
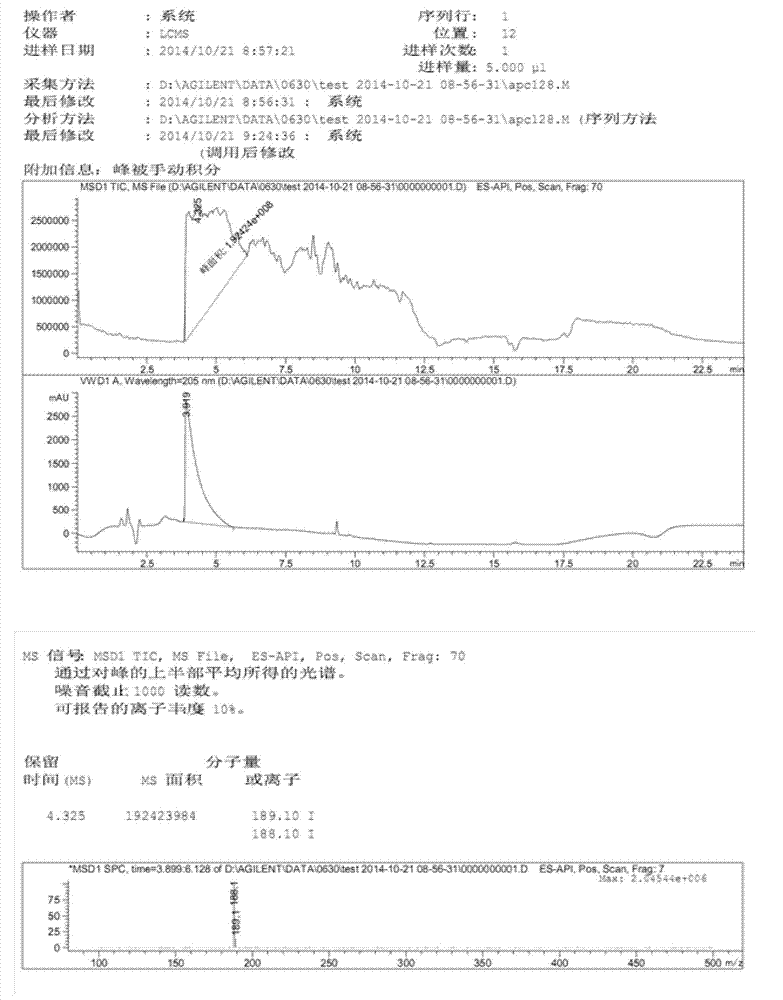
[0032] Ethyl di-o-tolyloxyphosphoryl acetate (4.29g, 12.3mmol) was dissolved in 40mL THF, cooled to -10°C under nitrogen protection. Potassium tert-butoxide (1.52g, 13.5mmol) was added in portions. After the addition was complete, the reaction was incubated for 2 hours. (2S)-3-tert-butyldimethylsilyloxy-2-methylpropanal (I) (2g, 9.9mmol) was configured into a THF solution with a mass percent concentration of 50%, and slowly added dropwise to the reaction in the liquid. After dropping, keep warm for 2 hours. After GC detection, the reaction was completed and quenched by adding water-saturated ammonium chloride aqueous solution. Separate the organic phase, remove the solvent, then add petroleum ether and water, extract and separate the layers. The aqueous phase was extracted twice with petroleum ether, and the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. Pave silica gel in sand core funnel, the solution after drying is s...
Embodiment 2
[0036]Dissolve ethyl di-2-tert-butylphenylphosphoryl acetate (23.3g, 53.9mmol) in 117mL THF, and lower the temperature to -15°C under nitrogen protection. Add potassium tert-butoxide (6.05g, 53.9mmol) in batches . After the addition was complete, the reaction was incubated for 1 hour. (2S)-3-tert-butyldimethylsilyloxy-2-methylpropanal (I) (8.4g, 41.5mmol) was configured into a THF solution with a mass percentage concentration of 20%, and slowly added dropwise to in the reaction solution. After dropping, keep warm for 3 hours. After GC detection, the reaction was completed and quenched by adding water-saturated ammonium chloride aqueous solution. Separate the organic phase, remove the solvent, then add petroleum ether and water, extract, and separate layers. The aqueous phase was extracted twice with petroleum ether, and the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. Pave silica gel in sand core funnel, the solution ...
Embodiment 3
[0040] Dissolve triethyl phosphoacetate (13.95 g, 62.2 mmol) in 100 mL of THF, and lower the temperature to -5°C under nitrogen protection. Add potassium tert-butoxide (1.52 g, 13.5 mmol) in portions. After the addition, the reaction was incubated for 2 hours. (2S)-3-tert-butyldimethylsilyloxy-2-methylpropanal (I) (9g, 44.5mmol) was configured into a THF solution with a mass percent concentration of 30%, and slowly added dropwise to the reaction in the liquid. After dropping, keep warm for 2 hours. After GC detection, the reaction was completed and quenched by adding water-saturated ammonium chloride aqueous solution. Separate the organic phase, remove the solvent, then add petroleum ether and water, extract and separate the layers. The aqueous phase was extracted twice with petroleum ether, and the organic phases were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. Pave silica gel in sand core funnel, the solution after drying is sucked and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com