Pharmaceutical-grade clean compressed air preparation system and method
A compressed air and preparation system technology, applied in separation methods, chemical instruments and methods, gas treatment, etc., can solve problems such as high oil content, low pass rate of bacterial endotoxin and microbial count detection, difficulty in meeting pharmaceutical grade requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation device of pharmaceutical grade clean compressed air:
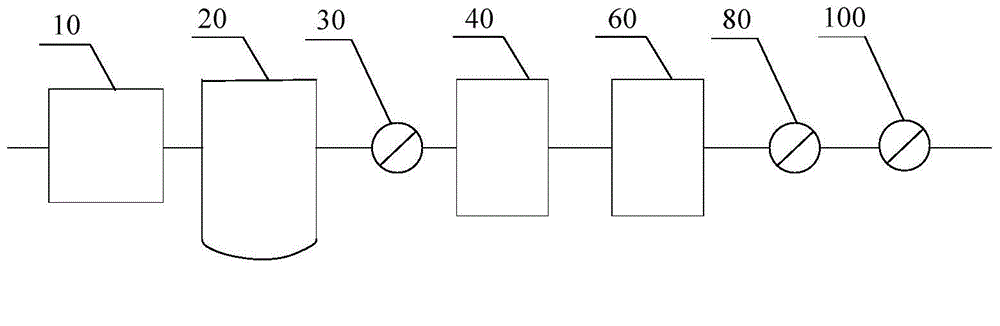
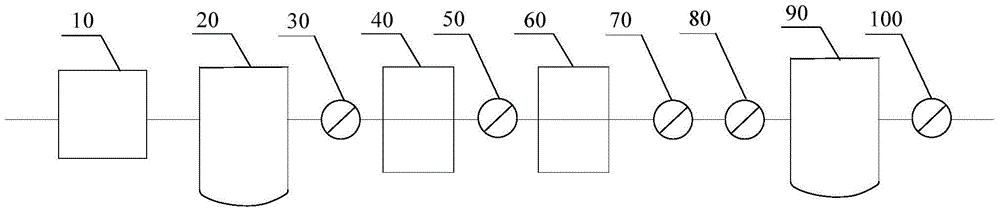
[0039] Such as figure 2 As shown, along the flow direction of the air, it includes an air compressor 10 (oil-free screw air compressor), a pressure buffer tank 20, a pre-filter 30 (filter aperture is 5 μm), a cooling dewatering machine 40, the first precision filter filter 50 (filter pore size 1 μm), adsorption drier 60 (adsorbent is activated alumina), second precision filter 70 (filter pore size 0.1 μm), activated carbon filter 80 (filter pore size 0.01 μm), pressure balance tank 90 and terminal filter 100 (filter pore size is 0.22 μm); and the components are connected by stainless steel pipelines.
[0040] Preparation method of pharmaceutical grade clean compressed air:
[0041] Introduce air into the preparation system of the above-mentioned pharmaceutical-grade clean compressed air, control the pressure of the compressed air output by the air compressor 10 to 0.78-0.96MPa, and adjust the temperat...
Embodiment 2
[0043] Preparation device of pharmaceutical grade clean compressed air:
[0044] Such as figure 2 As shown, along the flow direction of the air, it includes an air compressor 10 (oil-free screw air compressor), a pressure buffer tank 20, a pre-filter 30 (filter pore size is 4 μm), a cooling dewatering machine 40, the first precision filter device 50 (filter pore size is 0.5 μm), adsorption drier 60 (adsorbent is activated alumina), second precision filter 70 (filter pore size is 0.1 μm), activated carbon filter 80 (filter pore size is 0.01 μm), pressure The balance tank 90 and the final filter 100 (filter aperture is 0.22 μm); and the components are connected by stainless steel pipelines.
[0045] Preparation method of pharmaceutical grade clean compressed air:
[0046] Introduce air into the preparation system of the above-mentioned pharmaceutical-grade clean compressed air, control the pressure of the compressed air output by the air compressor 10 to 078-0.96MPa, adjust t...
Embodiment 3
[0048] Preparation device of pharmaceutical grade clean compressed air:
[0049] Such as figure 2 As shown, along the flow direction of the air, it includes an air compressor 10 (oil-free screw air compressor), a pressure buffer tank 20, a pre-filter 30 (filter pore size is 4 μm), a cooling dewatering machine 40, the first precision filter device 50 (filter pore size is 0.5 μm), adsorption drier 60 (adsorbent is activated alumina), second precision filter 70 (filter pore size is 0.1 μm), activated carbon filter 80 (filter pore size is 0.01 μm), pressure The balance tank 90 and the final filter 100 (filter aperture is 0.22 μm); and the components are connected by stainless steel pipelines.
[0050] Preparation method of pharmaceutical grade clean compressed air:
[0051] Introduce air into the preparation system of the above-mentioned pharmaceutical-grade clean compressed air, control the pressure of the compressed air output by the air compressor 10 to 0.8-0.95MPa, adjust t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com