A highly cohesive Pediococcus pentosaceus strain and its application in purifying Vibrio parahaemolyticus in water
A technology of Pediococcus pentosaceus and Vibrio hemolyticus, applied in the field of microorganisms, can solve the problems of food safety impact, drug-resistant bacteria and antibiotic residues, etc., and achieves strong cohesion and bacteriostatic properties, strong bacteriostatic properties, and application scope wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Bacterial Strain Recovery Culture
[0024] Thaw the seed preservation solution of Pediococcus pentosaceus at 0°C, aseptically draw 200 μL of the seed preservation solution and inoculate it into 10 mL of MRS liquid medium, culture it under anaerobic conditions at 37°C for 24 h, and take the culture to streak and inoculate in On the MRS solid plate, cultured anaerobically at 37°C for 24 h, then picked a single colony and inoculated in 5 mL of MRS liquid medium, cultured under anaerobic conditions at 37°C for 24 h, and set aside.
[0025] Inoculate the tdh gene-positive pathogenic Vibrio parahaemolyticus ATCC33847 in LBS liquid medium, shake and culture at 37°C for 16 h; take the culture to streak on the TCBS plate, culture at 37°C for 20 h, pick a single colony and inoculate 5 mL of LBS liquid medium, cultivated at 37°C for 12 h under constant temperature and shaking conditions, and set aside.
Embodiment 2
[0026] Embodiment 2 Determination of antibacterial ability
[0027] Comparative determination of antibacterial ability of Pediococcus pentosaceae F28-8, Y27-4, Y45-30, H13, H6, H10 and F3:
[0028] Centrifuge the culture of Pediococcus pentosaceae at 12,000g for 15 min at different temperatures of 70°C, 100°C, and 121°C respectively, take the supernatant, and sterilize it through a 0.22 µm microporous membrane to obtain a cell-free culture of Pediococcus pentosacea. extract. Use the agar well diffusion method to measure the antibacterial ability of the cell-free extract of the strain: Dilute the bacterial suspension of Vibrio parahaemolyticus to 10 with sterile physiological saline 8 CFU / mL, pipette 1 mL of bacterial suspension onto an LBS solid plate, spread it evenly, dry it in an ultra-clean workbench for 15 min, and use a puncher to uniformly punch small holes with a diameter of 10 mm in the plate, each hole Add 200 µL of Pediococcus pentosaceae culture cell-free extrac...
Embodiment 3
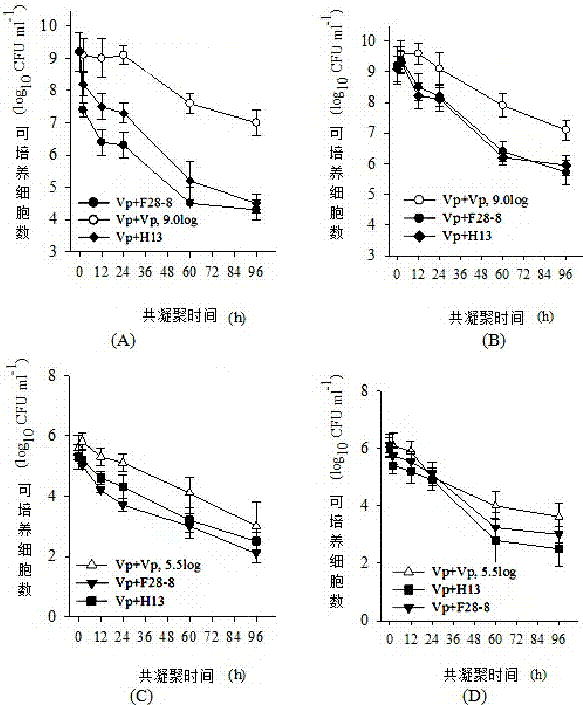
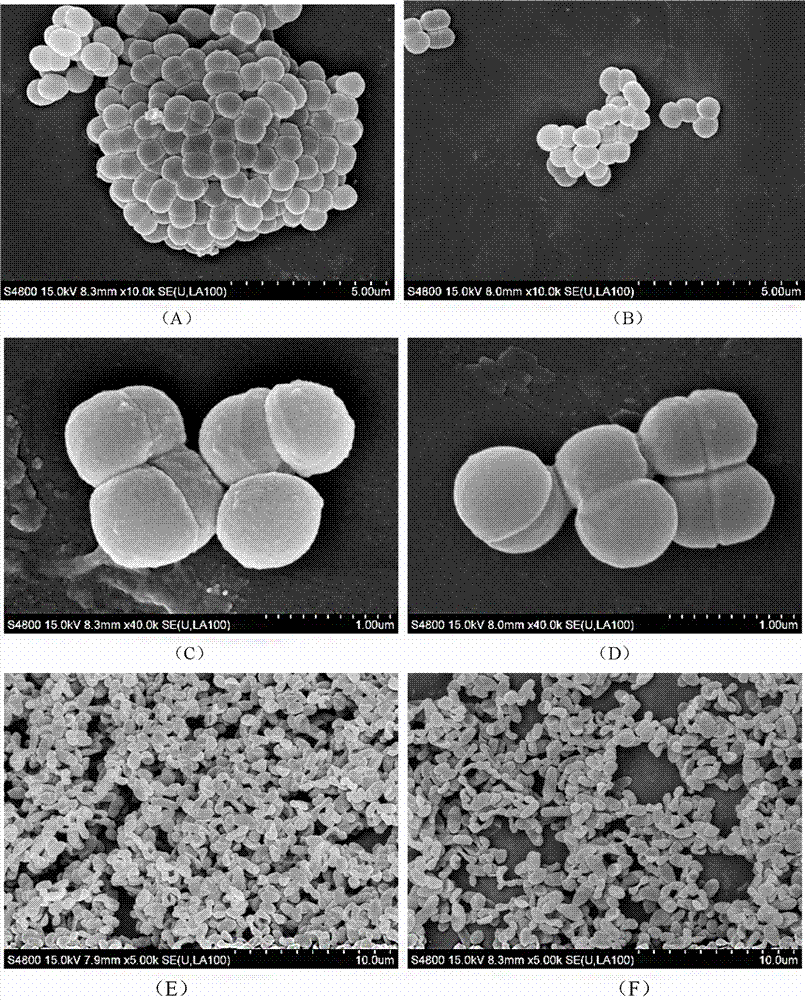
[0030] Example 3 Determination of Coagulation Ability
[0031] Comparative determination of coagulation ability of Pediococcus pentosaceae F28-8, Y27-4, Y45-30, H13, H6, H10 and F3:
[0032] Centrifuge the culture of Pediococcus pentosaceae strain at 5000g for 10 min, collect the cells, wash twice with normal saline, adjust the concentration of the suspension of the tested strain with normal saline, so that the absorbance value at 600 nm wavelength is about 0.4, that is, A600 =0.4. Mix 2 mL of the adjusted Pediococcus pentosaceae suspension and the corresponding Vibrio parahaemolyticus suspension in a 15 mL graduated test tube, vortex for 120 s, let stand at 37°C for 2 h, and draw 1 mL The absorbance value of the upper layer solution was measured at 600 nm, which was recorded as Amix. Three tubes of each mixed solution were repeated in parallel. The suspension of Vibrio parahaemolyticus without Pediococcus pentosaceae was used as a control to calculate the bacterial coagulati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com