Tilmicosin soluble powder and preparation method thereof
A soluble powder, tilmicosin technology, applied in powder delivery, pharmaceutical formulations, organic active ingredients and other directions, can solve the problems of complex preparation process, turbid solution, limited application, etc., and achieves good solubility, broad application prospects, and preparation. simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
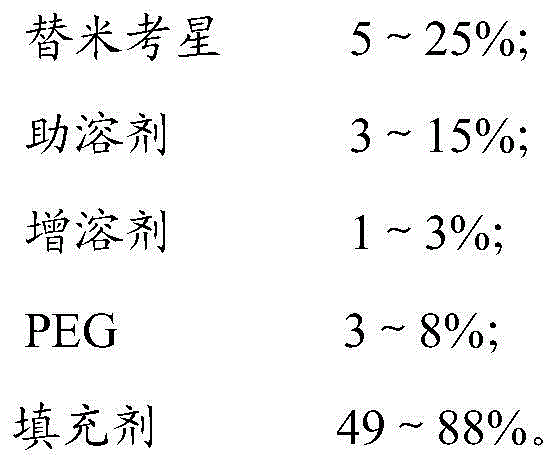
[0023] Weigh each raw material with the following mass percentage (take the total amount as 100g as an example):
[0024]
[0025] Take 5 g of tilmicosin, 3 g of sodium dihydrogen phosphate, 1 g of pluronic F68, 3 g of PEG4000 and 88 g of glucose, mix them evenly, and grind to obtain tilmicosin soluble powder.
[0026] The obtained tilmicosin soluble powder was tested according to the content specified in the national standard compilation of veterinary drugs, including: traits, identification, loss on drying, appearance uniformity, solubility, and content; the specific results are shown in Table 1.
Embodiment 2
[0028] Weigh each raw material with the following mass percentage (take the total amount as 100g as an example):
[0029]
[0030] Take 15g of tilmicosin, 3g of citric acid, 1g of pluronic F685gPEG6000 and 78g of glucose, mix evenly, and grind to obtain the soluble powder of tilmicosin.
[0031] The obtained tilmicosin soluble powder was detected by the detection method described in Example 1, and the specific results are shown in Table 1.
Embodiment 3
[0033]
[0034] Take 25g of tilmicosin, 15g of phosphoric acid, 3g of Tween-808gPEG6000 and 49g of glucose, mix evenly, and grind to obtain tilmicosin soluble powder.
[0035] The obtained tilmicosin soluble powder was detected by the detection method described in Example 1, and the specific results are shown in Table 1.
[0036] Table 1
[0037]
[0038]
[0039] It can be seen from Table 1 that the tilmicosin soluble powder provided by the present invention is a white powder, and each quality inspection index meets the compilation of national standards for veterinary drugs. It has high solubility, conforms to regulations, and has stable performance.
[0040] Adopt tilmicosin soluble powder of the present invention and preparation method thereof, its preparation process is simple, simply mixes and gets final product, and raw material is all commercially available raw material, and cheap and easy to obtain, and the performance of gained compound preparation is stable...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com