A crystal form of a proton pump inhibitor, its preparation intermediate, its synthesis method and its medical application
A crystal form and drug technology, which is applied in the preparation of carboxylate salts, active ingredients of heterocyclic compounds, drug combinations, etc., can solve problems such as ulcers, and achieve the effect of stable crystal form properties and good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
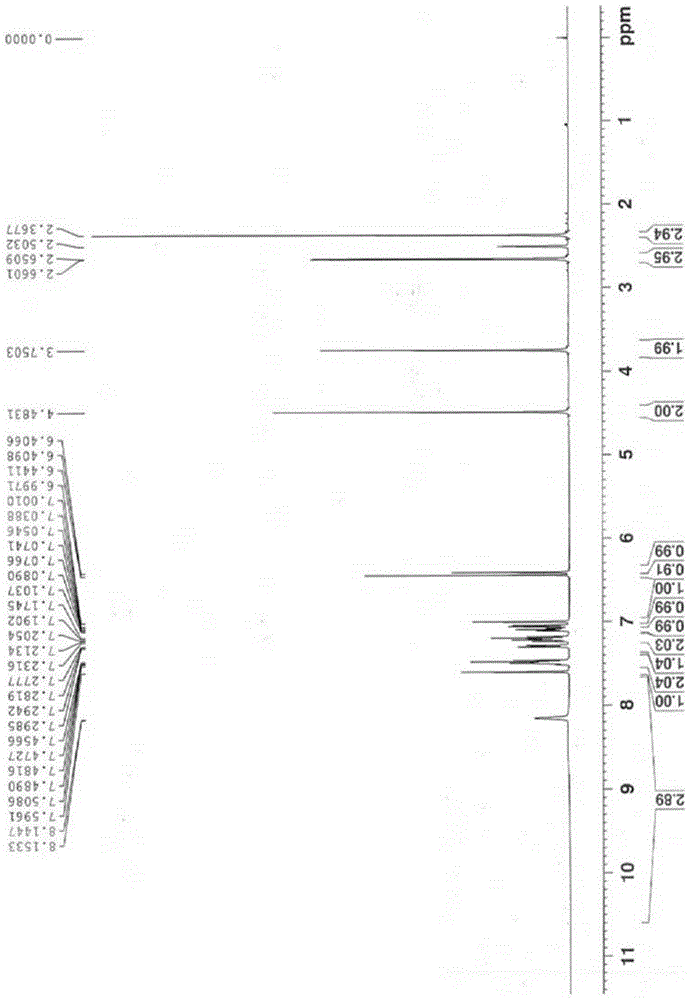
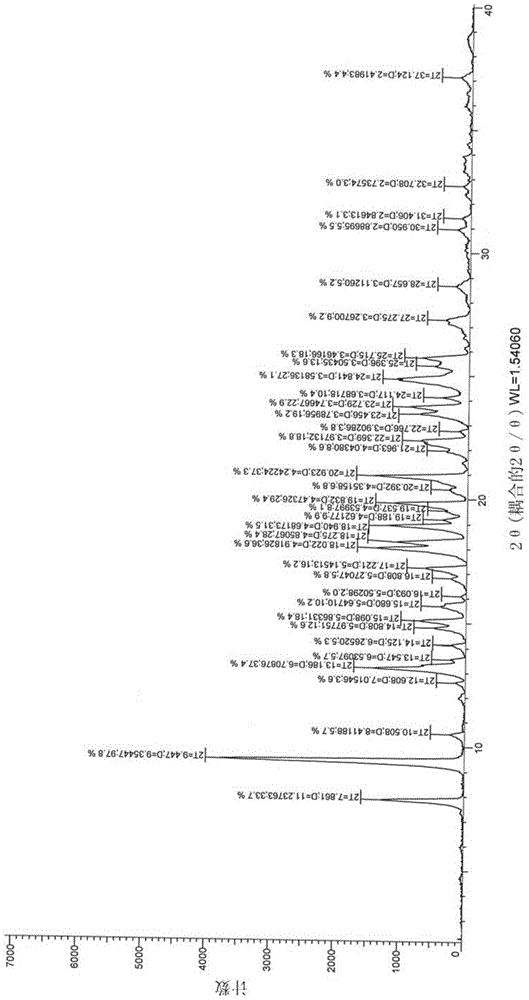
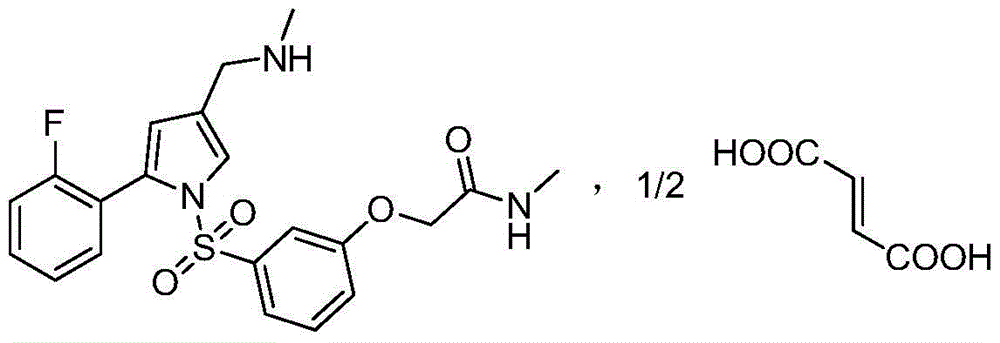
[0036] Example 1: 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)- N-methylacetamide 10g, be dissolved in the mixed solvent of isopropanol (80ml) and absolute ethanol (20ml), add fumaric acid 1.26g under the condition of stirring, then heat to 50 ℃ and reflux for 30 minutes, in Naturally cooled to room temperature with stirring, stirred for 2 hours, filtered, and dried to obtain 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrole- 1-yl)sulfonyl)phenoxy)-N-methylacetamide hemifumarate, the target crystal form was obtained, named as crystal form 1, weighing 9.8g, mass yield 98%, its 1H-NMR Spectrum such as figure 1 As shown, the XRD pattern is as figure 2 shown.
Embodiment 2
[0037] Example 2: 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)- 10g of N-methylacetamide, dissolved in isopropanol (80ml), added ethanol solution of fumaric acid (1.26g / 20ml), heated to reflux, all solids dissolved, naturally cooled to room temperature under stirring conditions, stirred for 2 hours, filtered and dried to give 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy) -N-methylacetamide hemifumarate, the target crystal form was obtained, named as crystal form 1, weighed 9.6g, and the mass yield was 96%. Its 1H-NMR spectrum was basically the same as figure 1 The same, the XRD pattern is basically the same as figure 2 Consistent with the characteristics of crystal form 1.
Embodiment 3
[0038]Example 3: 2-(3-((2-(2-fluorophenyl)-4-((methylamino)methyl)-1H-pyrrol-1-yl)sulfonyl)phenoxy)-N -Preparation of methylacetamide hemifumarate intermediate and free base
[0039]
[0040] first step
[0041] tert-Butyl((5-(2-fluorophenyl)-1H-pyrrol-3-yl)methyl)(methyl)carbonate (3.0g, 10mmol) and 3-(2-(methylamino)- Add 2-oxoethoxy)phenyl-1-sulfonyl chloride (2.64mg, 10mmol) into acetonitrile, add DIEA and stir for 2-5 hours. The temperature of the reaction solution was cooled, dilute hydrochloric acid was added to adjust the pH to 4-5, and purified water was added for crystallization to obtain compound III, 4.9 g, yield: 92.3%.
[0042] second step
[0043] Compound III (4.5 g) was dissolved in 15 mL of ethyl acetate, the temperature of the reaction liquid was cooled to 10° C. in an ice bath, hydrochloric acid gas was added, and the reaction was stirred for 1 hour. Use sodium bicarbonate to adjust pH to alkaline, wash with saturated sodium chloride solution, dry ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com