Intraocular tension reducing compound and preparation method and use thereof
A technology for compounds and analogs, applied in the field of preparing medicines for reducing intraocular pressure, can solve problems such as unsatisfactory treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
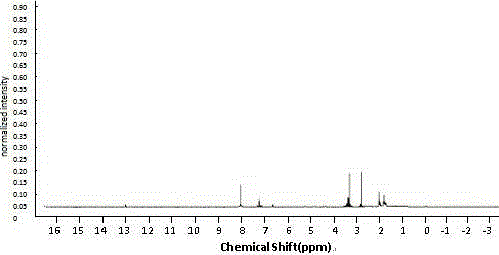
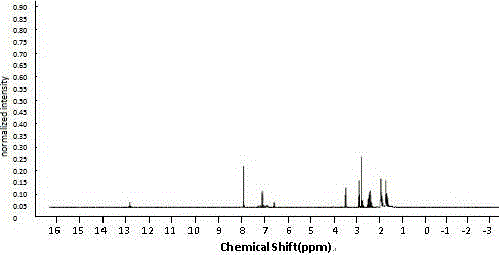
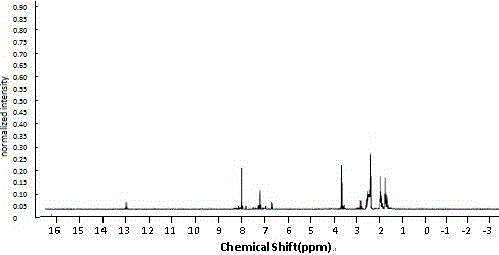
Image
Examples
preparation Embodiment 1
[0031] Preparation Example 1 (compound A is the preparation of compound A11)
[0032] Compound A1 and A2 are combined to generate compound A3, A3 is brominated to obtain compound A4, benzene is substituted for bromine to generate compound A5, deaminated to generate compound A6, nitro is substituted to generate compound A7, nitro is reduced to generate compound A8, under the protection of Boc, The unprotected amino group of A8 is activated to generate compound A9, the de-Boc of trifluoroacetic acid is generated to generate A10, and the cycloalkylamino group is activated to generate A11.
[0033]
preparation Embodiment 2
[0034] Preparation Example 2 (compound B is the preparation of compound B11)
[0035] Compound B1 and B2 are combined to generate compound B3, B3 is brominated to obtain compound B4, benzene is substituted for bromine to generate compound B5, deaminated to generate compound B6, nitro is substituted to generate compound B7, nitro is reduced to generate compound B8, under the protection of Boc, The unprotected amino group of B8 is activated to generate compound B9, the de-Boc of trifluoroacetic acid generates B10, and the cycloalkylamino group is activated to generate B11.
[0036]
preparation Embodiment 3
[0037] Preparation Example 3 (Preparation of Compound C, Compound C11)
[0038] Compound C1 and C2 are combined to generate compound C3, C3 is brominated to obtain compound C4, benzene is substituted for bromine to generate compound C5, deaminated to generate compound C6, nitro is substituted to generate compound C7, nitro is reduced to generate compound C8, under the protection of Boc, C8 The amino group not protected by Boc is activated to generate compound C9, the de-Boc of trifluoroacetic acid is generated to generate C10, and the cycloalkylamino group is activated to generate C11.
[0039]
[0040] Preparation of injection containing compound A:
[0041] ① Take mannitol, phospholipids, glycerol, cyclodextrin derivatives, dimethyl sulfoxide and poloxamer, 50mg and 100mg of the compound of formula (A), mix them in water for injection and dissolve them;
[0042] ② After mixing and dissolving, after stabilization, first use a 0.45um microporous membrane for coarse f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com