Use of a semiconducting compound in an organic light emitting device
A compound and application technology, applied in the application field of semiconductor compounds in organic light-emitting devices, can solve problems such as unclear thermal stability, and achieve the effect of high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
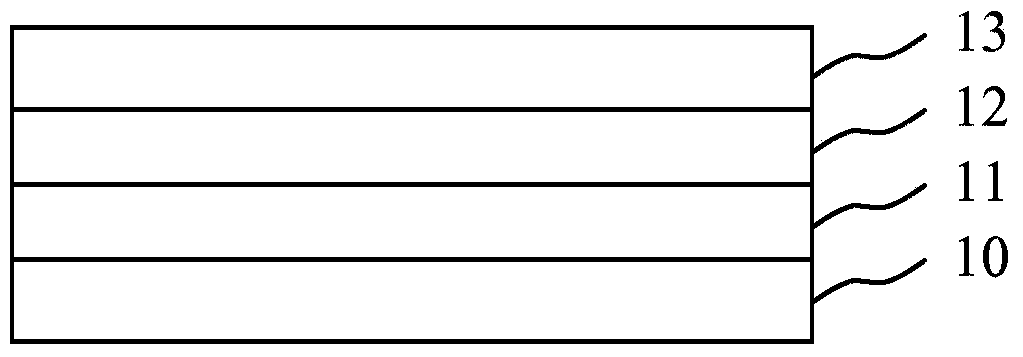
[0206] Bottom blue emitting OLEDs were fabricated on glass substrates coated with patterned ITO (90 nm thickness) with the following layer sequence:
[0207] 1. p-doped N,N'-bis(naphthalene-1-yl)-N,N'-bis(phenyl)-benzidine (a-NPD) (5 mol% of 2,2',2 ″-(cyclopropane-1,2,3-triylidene) tris(2-(p-cyanotetrafluorophenyl)acetonitrile) (PD2) is used as a hole injection and transport layer with a thickness of 130nm;
[0208] 2. Undoped 4,4',4"-tris(carbazol-9-yl)-triphenylamine (TCTA), with a thickness of 10nm;
[0209] 3. Emitter layer with TPBI:Firpic (molar ratio 4:1) with a thickness of 15 nm. TPBI is 1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene and Firpic is bis(3,5-difluoro-2-(2-pyridyl-(2-carboxy pyridyl) iridium (III);
[0210] 4.20nm TPBI;
[0211] 5.40nm tetrakis(1,3,4,6,7,8-hexahydro-2H-pyrimido[1,2-a]pyrimidinate) ditungsten(II)(W 2 (hpp) 4 ) doped compound C1 (70:30 mol%);
[0212] 6. Cathode of 100nm Al.
[0213] Encapsulate the device with a cover glass layer...
Embodiment 2
[0215] A second OLED was fabricated as described in Example 1, except that compound C2 was used instead of compound C1. at 10mA / cm 2 The voltage at 4.3V and the device reaches 1000cd / m at 4.8V 2 .
Embodiment 3
[0217] A third OLED was fabricated as described in Example 1, except that Compound C3 was used instead of Compound Cl. at 10mA / cm 2 The voltage at 4.0V and the device reaches 1000cd / m at 4.2V 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com